Abstract
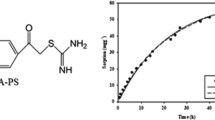
In the present study, a Schiff’s base polymer (SBP) has been synthesized by the condensation of trifluoroacetylacetone and amino-polystyrene copolymer resin. After the characterization of synthesized polymer by FT-IR spectroscopy, thermal and elemental analyses, the removal of 2-chlorophenol (2-CP), 2,4-dichlorophenol (2,4-DCP) along with phenol is optimized using response surface methodology. Eighteen runs design were performed with different levels of variables in order to optimize sorption parameters such as pH, concentration of adsorbate, agitation time and amount of polymeric resin SBP. Maximum Langmuir sorption capacity obtained was 110.7, 125.3 and 195.4 mg g−1, for phenol, 2-CP and 2,4-DCP, respectively. Recovery of adsorbed phenol, 2-CP and 2,4-DCP was checked by different solvents and was found to be quantitative (~ 99%) with methanol. The model was validated by performing sorption experiments at optimum conditions. Kinetic and isotherm studies of all three analytes on SBP are also discussed.







Similar content being viewed by others
References
Zahangir A, Suleyman A, Juria M-T (2007) Statistical optimization of adsorption processes for removal of 2,4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. J Environ Sci 19:674–677
Zhang L, Zhang B, Wu T, Sun D (2015) Adsorption behavior and mechanism of chlorophenols onto organoclays in aqueous solution. Colloids Surf A Physicochem Eng Asp 484:118–129
Peredo-Mancilla D, Dominguez H (2016) Adsorption of phenol molecules by sodium dodecyl sulfate (SDS) surfactants deposited on solid surfaces. A computer simulation study. J Mol Graph Model 65:108–112
Garba A, Nasrib N-S, Basria H, Ismaild R, Majid Z-A, Hamza U-D, Mohammed J (2016) Adsorptive removal of phenol from aqueous solution on a modified palm shell-based carbon: fixed-bed adsorption studies. Desalin Water Treat 57:29488–29499
Chaudhary N, Balomajumder C (2014) Optimization study of adsorption parameters for removal of phenol on aluminum impregnated fly ash using response surface methodology. J Taiwan Inst Chem Eng 45:852–859
Vargas D-P, Giraldo L, Moreno-Piraján J-C (2017) Effect of textural and chemical characteristics of activated carbons on phenol adsorption in aqueous solutions. Pol J Chem Technol 19(4):87–93
Huang J, Zha H, Jin X, Deng S (2012) Efficient adsorptive removal of phenol by a diethylenetriamine-modified hyper crosslinked styrene–divinylbenzene (PS) resin from aqueous solution. Chem Eng J 195:40–48
Kalderis D, Koutoulakis D, Paraskeva P, Diamadopoulos E, Otal E, Valle J-O, Fernandez-Pereira C (2008) Adsorption of polluting substances on activated carbons prepared from rice husk and sugarcane bagasse. Chem Eng J 144:42–50
Qiu Y-P, Cheng H-Y, Xu C, Sheng D (2008) Surface characteristics of crop-residue derived black carbon and lead(II) adsorption. Water Res 42:567–574
Ipek I, Kabay N, Yuksel M (2017) Separation of bisphenol A and phenol from water by polymer adsorbents: equilibrium and kinetics studies. J Water Process Eng 16:206–211
Zeng X, Tingjun Y, Wang P, Yuan R, Wen Q, Fan Y, Wang C, Shi R (2010) Preparation and characterization of polar polymeric adsorbents with high surface area for the removal of phenol from water. J Hazard Mater 177:773–780
Xiao G, Wen R, Wu P, You D (2017) Adsorption of phenol onto four hyper-cross-linked polymeric adsorbents: effect of hydrogen bonding receptor in micropores on; adsorption capacity. Microporous Mesoporous Mater 239:40–44
Pacurariu C, Mihoc G, Popa A, Muntean S-G, Ianos R (2013) Adsorption of phenol and p-chlorophenol from aqueous solutions on poly (styrene-co-divinylbenzene) functionalized materials. Chem Eng J 222:218–227
Caetano M, Valderrama C, Farran A, Luis L-C (2009) Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins. J Colloid Interface Sci 338:402–409
Li A, Zhang Q, Zhang G, Chen J, Fei Z, Liu F (2002) Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 47:981–989
Zhang W-M, Chen J-L, Pan B-C, Zhang Q-X, Zhang B (2006) Synergistic adsorption of phenol from aqueous solution onto polymeric adsorbents. J Hazard Mater B128:123–129
Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S (2009) Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J 151:19–29
Leon-Gonzalez M-E, Perez-Arribas L-V (2000) Chemically modified polymeric sorbents for sample preconcentration. J Chromatogr A 902:3–16
Ayranci E, Conway B-E (2001) Removal of phenol, phenoxide and chlorophenols from waste-waters by adsorption and electrosorption at high-area carbon felt electrodes. J Electroanal Chem 513:100–110
Memon S-Q, Hasany S-M, Bhanger M-I, Khuhawar M-Y (2005) Enrichment of Pb(II) ions using phthalic acid functionalized XAD-16 resin as a sorbent. J Colloid Interface Sci 291:84–91
Saad M, Tahir H, Khan J, Hameed U, Saud A (2017) Synthesis of polyaniline nanoparticles and their application for the removal of crystal violet dye by ultrasonicated adsorption process based on response surface methodology. Ultrason Sonochem 34:600–608
Jung Y, Koh H, Shin W, Sung N (2005) Wastewater treatment using combination of MBR equipped with non-woven fabric filter and oyster-zeolite column. Environ Eng Res 10:247–256
Qureshi T, Memon N, Memon S-Q, Ashraf M-A (2015) Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin Water Treat 57(1):1–9
Channa A-M, Siyal A-N, Memon S-Q, Parveen S (2016) Design of experiment for treatment of arsenic-contaminated water using Schiff’s base metal complex modified Amberlite XAD-2. Desalin Water Treat 57(8):1–8
Siyal A-N, Memon S-Q, Mahar A, Pirzada T, Parveen S, Sodho N-A (2013) Multi-variant sorption optimization for the uptake of Pb(II) ions by Jamun Seed Waste. Pol J Chem Technol 15(1):15–21
Fierro V, Torne Fernandez V, Montane D, Celzard A (2008) Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater 111:276–284
Shokoohi R, Saghi M-H, Ghafari H-R, Hadi M (2009) biosorption of iron from aqueous solution by dried biomass of activated sludge. Iran J Environ Health Sci Eng 6(2):107–111
Acknowledgements
Authors are highly grateful to Institute of Advanced Research Studies in Chemical Sciences for providing laboratory facilities to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soomro, F.K., Memon, S.Q., Memon, N. et al. A new Schiff’s base polymer for remediation of phenol, 2-chlorophenol and 2,4-dichlorophenol from contaminated aqueous systems. Polym. Bull. 77, 2367–2383 (2020). https://doi.org/10.1007/s00289-019-02852-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02852-6