Abstract
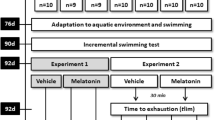
Exercise induces different effects on antioxidant status depending on its intensity. The forced running wheel (FRW) model maintains a constant intensity and volume during exercise. The aim of the present study was to investigate the effects of FRW exercise at different running speeds on several serum biochemical parameters of liver and muscle functions and on oxidative stress biomarkers in skeletal muscle, liver and serum in the rat. Thirty-six male Wistar rats were randomly divided into six groups. Five groups participated in constant power tests at intensities of 10, 13, 14.5, 16, and 17.5 m/min, and a non-exercise group was chosen as the control. Serum, muscle and liver tissues were collected after the tests and analyzed. At speeds >16 m/min, exercise on an FRW significantly increased several serum biochemical parameters, malondialdehyde level and superoxide dismutase activity in all tissues of exercise rats compared with control rats; FRW exercise also increased catalase activity in the liver and glutathione S-transferase activity in muscle, whereas it decreased glutathione level in all tissues and catalase activity in muscle and serum. These data suggest that FRW exercise in rats activates an adaptation of the antioxidant system response in skeletal muscle at speeds <16 m/min, whereas it induces oxidative stress at higher speeds in muscle, liver and serum. In addition, we observed a correlation between the systematic and local oxidative stress status in rats after exercise on FRW.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gradari, S., Palle, A., McGreevy, K. R., Fontan-Lozano, A. & Trejo, J. L. Can exercise make you smarter, happier, and have more neurons? A hormetic perspective. Front. Neurosci. 10, 93 (2016).
Guerreiro, L. F. et al. Oxidative status of the myocardium in response to different intensities of physical training. Physiol. Res. 65, 737–749 (2016).
Emami, S. R., Jafari, M., Haghshenas, R. & Ravasi, A. Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. J. Exerc. Nutrition Biochem. 20, 29–35 (2016).
Vina, J. et al. Free radicals in exhaustive physical exercise: mechanism of production, and protection by antioxidants. IUBMB Life 50, 271–277 (2000).
Naderi, R. et al. Voluntary exercise protects heart from oxidative stress in diabetic rats. Adv. Pharm. Bull. 5, 231–236 (2015).
Selman, C., McLaren, J. S., Collins, A. R., Duthie, G. G. & Speakman, J. R. Antioxidant enzyme activities, lipid peroxidation, and DNA oxidative damage: the effects of short-term voluntary wheel running. Arch. Biochem. Biophys. 401, 255–261 (2002).
Berzosa, C. et al. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. J. Biomed. Biotechnol. 2011, 540458 (2011).
Morillas-Ruiz, J. M. & Hernández-Sánchez, P. Oxidative stress and antioxidant defenses induced by physical exercise. In Basic Principles and Clinical Significance of Oxidative Stress (ed. Gowder, S. J. T.) 221–241 (IntechOpen, 2015). Available from https://www.intechopen.com/books/basic-principles-and-clinical-significance-of-oxidative-stress/oxidative-stress-and-antioxidant-defenses-induced-by-physical-exercise.
Buresh, R. & Berg, K. A tutorial on oxidative stress and redox signaling with application to exercise and sedentariness. Sports Med. Open 1, 3 (2015).
Radak, Z. et al. The redox-associated adaptive response of brain to physical exercise. Free Radic. Res. 48, 84–92 (2014).
Powers, S. K. & Jackson, M. J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276 (2008).
Henriquez-Olguín, C. et al. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 24, 101188 (2019).
Toval, A. et al. Habituation training improves locomotor performance in a forced running wheel system in rats. Front. Behav. Neurosci. 11, 42 (2017).
Rezaei, S. et al. Evaluation of efforts in untrained Wistar rats following exercise on forced running wheel at maximal lactate steady state. J. Exerc. Nutrition Biochem. 21, 26–32 (2017).
Leasure, J. L. & Jones, M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156, 456–465 (2008).
Meijer, J. H. & Robbers, Y. Wheel running in the wild. Proc. Biol. Sci. 281, 20140210 (2014).
Manchado, F. et al. The maximal lactate steady state is ergometer-dependent in experimental model using rats. Rev. Bras. Med. Esporte 12, 259–262 (2006).
Khazaie, S., Jafari, M., Heydari, J. & Salem, F. Investigating response of spleen and erythrocytes antioxidant defense system on the effects of n-acetyl cysteine against Paraoxon toxicity in rat. Stud. Med. Sci. 26, 176–184 (2015).
Ferreira, J. C. et al. Maximal lactate steady state in running mice: effect of exercise training. Clin. Exp. Pharmacol. Physiol. 34, 760–765 (2007).
Contarteze, R. V., Manchado, F., de, B., Gobatto, C. A. & De Mello, M. A. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 151, 415–422 (2008).
Jafari, M. et al. Effects of paraoxon on serum biochemical parameters and oxidative stress induction in various tissues of Wistar and Norway rats. Environ. Toxicol. Pharmacol. 34, 876–887 (2012).
Khazaie, S. et al. Modulatory effects of vitamin C on biochemical and oxidative changes induced by acute exposure to diazinon in rat various tissues: prophylactic and therapeutic roles. J. Anim. Physiol. Anim. Nutr. 103, 1619–1628 (2019).
Clarkson, P. M., Kearns, A. K., Rouzier, P., Rubin, R. & Thompson, P. D. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med. Sci. Sports Exerc. 38, 623–627 (2006).
Poprzecki, S., Staszkiewicz, A. & Hubner-Wozniak, E. Effect of eccentric and concentric exercise on plasma creatine kinase (CK) and lactate dehydrogenase (LDH) activity in healthy adults. Biol. Sport 21, 193–202 (2004).
Pettersson, J. et al. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 65, 253–259 (2008).
Zhonghui, Z., Xiaowei, Z. & Fang, F. Ganoderma lucidum polysaccharides supplementation attenuates exercise-induced oxidative stress in skeletal muscle of mice. Saudi J. Biol. Sci. 21, 119–123 (2014).
Forman, H. J., Ursini, F. & Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 73, 2–9 (2014).
Ayala, A., Muñoz, M. F. & Argüelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 360438 (2014).
Lin, X., Qu, S., Hu, M. & Jiang, C. Protective effect of erythropoietin on renal injury induced by acute exhaustive exercise in the rat. Int. J. Sports Med. 31, 847–853 (2010).
Lima, M. C. et al. Evaluation of oxidative stress in mice subjected to aerobic exercise. Acta Cir. Bras. 27, 544–551 (2012).
Acikgoz, O., Aksu, I., Topcu, A. & Kayatekin, B. M. Acute exhaustive exercise does not alter lipid peroxidation levels and antioxidant enzyme activities in rat hippocampus, prefrontal cortex and striatum. Neurosci. Lett. 406, 148–151 (2006).
Bejma, J. & Ji, L. L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 87, 465–470 (1999).
Vukovic, R. et al. Impact of ovariectomy, high fat diet, and lifestyle modifications on oxidative/antioxidative status in the rat liver. Croat. Med. J. 55, 218–227 (2014).
Belviranlı, M. & Gökbel, H. Acute exercise induced oxidative stress and antioxidant changes. Eur. J. Gen. Med. 3, 126–131 (2006).
Jackson, M. J. Reactive oxygen species and redox-regulation of skeletal muscle adaptations to exercise. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 2285–2291 (2005).
Holloway, T. M., Bloemberg, D., da Silva, M. L., Quadrilatero, J. & Spriet, L. L. High-intensity interval and endurance training are associated with divergent skeletal muscle adaptations in a rodent model of hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R927–R934 (2015).
Wang, P., Li, C. G., Qi, Z., Cui, D. & Ding, S. Acute exercise induced mitochondrial H2O2 production in mouse skeletal muscle: association with p66Shc and FOXO3a signaling and antioxidant enzymes. Oxid. Med. Cell. Longev. 2015, 536456 (2015).
Gomes, M. J. et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget 8, 20428–20440 (2017).
Vargas-Mendoza, N. et al. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants 8, 196 (2019).
Sallam, N. & Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell. Longev. 2016, 7239639 (2016).
Kerksick, C. & Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2, 38–44 (2005).
Kayatekin, B. M., Gönenç, S., Açikgöz, O., Uysal, N. & Dayi, A. Effects of sprint exercise on oxidative stress in skeletal muscle and liver. Eur. J. Appl. Physiol. 87, 141–144 (2002).
Ji, L. L. Exercice-induced oxidative stress in the heart. In Handbook of Oxidants and Antioxidants in Exercise (eds. Sen, C. K., Packer, L. & Hänninen, O. O. P.) 689–712 (Elsevier Science, Amsterdam, Netherlands, 1994).
Margaritelis, N. V. et al. Blood reflects tissue oxidative stress: a systematic review. Biomarkers 20, 97–108 (2015).
Veskoukis, A. S., Nikolaidis, M. G., Kyparos, A. & Kouretas, D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 47, 1371–1374 (2009).
da Rocha, R. F. et al. Vascular redox imbalance in rats submitted to chronic exercise. Cell Biochem. Funct. 28, 190–196 (2010).
Leeuwenburgh, C. & Ji, L. L. Glutathone and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exercise. J. Nutr. 128, 2420–2426 (1998).
Veskoukis, A. S. et al. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl. Physiol. Nutr. Metab. 33, 1140–1154 (2008).
You, T. et al. Oxidative stress response in normal and antioxidant supplemented rats to a downhill run: changes in blood and skeletal muscles. Can. J. Appl. Physiol. 30, 677–689 (2005).
Ramos, D., Martins, E. G., Viana-Gomes, D., Casimiro-Lopes, G. & Salerno, V. P. Biomarkers of oxidative stress and tissue damage released by muscle and liver after a single bout of swimming exercise. Appl. Physiol. Nutr. Metab. 38, 507–511 (2013).
Tanner, R. K., Fuller, K. L. & Ross, M. L. Evaluation of three portable blood lactate analysers: lactate pro, lactate scout and lactate plus. Eur. J. Appl. Physiol. 109, 551–559 (2010).
Winterbourn, C. C., Hawkins, R. E., Brian, M. & Carrell, R. W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 85, 337–341 (1975).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).
Habig, W. H. & Jakoby, W. B. Glutathione S-transferases (rat and human). Methods Enzymol. 77, 218–231 (1981).
Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27, 502–522 (1969).
Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 90, 37–43 (1978).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Acknowledgements
The authors would like to thank Javad Rasouli from Baqiyatallah University of Medical Sciences for his assistance. This work was supported by a grant from Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
S.R.M. performed the experiments; M.J. designed the study, contributed to the methodology, wrote, reviewed and edited the manuscript; S.R. performed the experiments and statistical analysis; H. A-a designed the study and provided resources; V. S. contributed to the methodology.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mousavi, S.R., Jafari, M., Rezaei, S. et al. Evaluation of the effects of different intensities of forced running wheel exercise on oxidative stress biomarkers in muscle, liver and serum of untrained rats. Lab Anim 49, 119–125 (2020). https://doi.org/10.1038/s41684-020-0503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-020-0503-7