Abstract
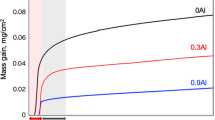
The high-temperature oxidation behavior of Ni–11Fe–10Cu–6Al alloy at 750, 850 and 950 °C in air was investigated. Its oxidation kinetics follow the parabolic rate law at all temperatures and are significantly lower than those of Ni–11Fe–10Cu and Ni–11Fe–10Cu–6Al–3Y alloys. The doping of Al to Ni–11Fe–10Cu alloy can improve not only the adherence of oxide scale but also the oxidation resistance. The structure and growth mechanism of multilayer oxide scale formed on Ni–11Fe–10Cu–6Al alloy are studied. The effect of doping Al on the high-temperature oxidation behavior of Ni–11Fe–10Cu alloy is also discussed.
Graphic Abstract












Similar content being viewed by others
References
K. Le Van, H. Groult, F. Lantelme, M. Dubois, D. Avignant, A. Tressaud, S. Komaba, N. Kumagai and S. Sigrist, Electrochimica Acta54, 2009 (4566).
S. Helle, B. Brodu, B. Davis, D. Guay and L. Roué, Corrosion Science53, 2011 (3248).
L. Cassayre, P. Palau, P. Chamelot and L. Massot, Journal of Chemical & Engineering Data55, 2010 (4549).
X. H. Cheng, L. Fan, H. Y. Yin, L. Liu, K. F. Du and D. H. Wang, Corrosion Science112, 2016 (54).
X. H. Cheng, H. Y. Yin and D. H. Wang, Corrosion Science141, 2018 (168).
H. Y. Yin, D. Y. Tang, H. Zhu and D. H. Wang, Electrochemistry Communications13, 2011 (1521).
D. Y. Tang, H. Y. Yin, X. H. Cheng, W. Xiao and D. H. Wang, International Journal of Hydrogen Energy41, 2016 (18699).
X. H. Cheng, D. Y. Tang, D. D. Tang, H. Zhu and D. H. Wang, Journal of The Electrochemical Society162, 2015 (E68).
S. J. Park, S. M. Seo, Y. S. Yoo, H. W. Jeong and H. Jang, Corrosion Science90, 2015 (305).
J. N. Hryn and M. J. Pellin, Light Metals, 1999 (377).
J. N. Hryn and D. R. Sadoway, Light Metals, 1993 (475).
M. Glucina and M. Hyland, Corrosion Science48, 2006 (2457).
S. Hayashi, S. Narita and T. Narita, Oxidation of Metals66, 2006 (191).
S. C. Choi, H. J. Cho, Y. J. Kim and D. B. Lee, Oxidation of Metals46, 1996 (51).
J. Klöwer, U. Brill and U. Heubner, Intermetallics7, 1999 (1183).
Y. C. Ma, X. J. Zhao, M. Gao and K. Liu, Journal of Materials Science & Technology27, 2011 (841).
V. Chapman, B. J. Welch and M. Skyllas-Kazacos, Electrochimica Acta56, 2011 (1227).
X. H. Cheng, L. Fan, L. Liu, K. F. Du and D. H. Wang, Journal of Rare Earths34, 2016 (1139).
R. Peraldi, D. Monceau and B. Pieraggi, Oxidation of Metals58, 2002 (249).
J. F. Moore, M. P. McCann, M. J. Pellin, A. Zinovev and J. N. Hryn, Journal of Vacuum Science & Technology A21, 2003 (1631).
Y. B. Zhou and H. J. Zhang, Transactions of Nonferrous Metals Society of China22, 2012 (2041).
R. Haugsrud, T. Norby and P. Kofstad, Corrosion Science43, 2001 (283).
R. Haugsrud and P. Kofstad, Oxidation of Metals50, 1998 (189).
R. Haugsrud, Oxidation of Metals55, 2001 (571).
M. T. Shim and W. J. Moore, The Journal of Chemical Physics26, 1957 (802).
B. Amami, M. Addou, F. Millot, A. Sabioni and C. Monty, Ionics5, 1999 (358).
M. H. Li, X. F. Sun, J. G. Li, Z. Y. Zhang, T. Jin, H. R. Guan and Z. Q. Hu, Oxidation of Metals59, 2003 (591).
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (Grant No. 51874211) and the Key Program of the Scientific Research Foundation of the Education Bureau of Hubei Province, China (Grant No. D20181901).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, X., Du, K. & Wang, D. Effect of Doping Al on the High-Temperature Oxidation Behavior of Ni–11Fe–10Cu Alloy. Oxid Met 93, 417–431 (2020). https://doi.org/10.1007/s11085-020-09963-w
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-020-09963-w