Abstract
Objectives
We evaluated the effects of low-level laser therapy (LLLT) using an infrared laser (IRL) and a red laser (RL) on the pulp of molar teeth in rats after dental bleaching to assess inflammation, collagen fiber maturation, and tertiary dentin formation.
Materials and methods
Eighty Wistar rats (Rattus norvegicus, albinus) were randomly divided into eight groups with 10 hemimaxillae in each of the following: control; bleached (Ble, 35% hydrogen peroxide [H2O2]); Ble-1IRL and Ble-1RL (one IRL [808 nm, 30 s, 3 J] or RL [660 nm, 15 s, 1.5 J] application immediately after H2O2); Ble-3IRL and Ble-3RL (three [immediately, 24 h, and 48 h] IRL or RL applications after H2O2); and 3IRL and 3RL (three IRL or RL applications without bleaching). The rats were euthanized after 2 and 30 days for histological evaluation of inflammation (hematoxylin-eosin) and maturation of collagen fibers (picrosirius red). Additionally, the dentin deposition in the specimens obtained at 30 days was quantified via microtomography of the pulp chamber volume. Statistical analyses were performed (P < 0.05).
Results
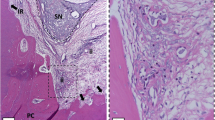
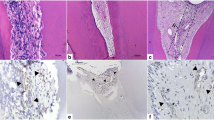
Initially, severe damages to the pulp were observed in the Ble and Ble-1RL groups. Ble-1IRL and Ble-3RL groups showed lower inflammation. The bleached groups had a greater amount of mature collagen fibers than the control group. The Ble-3IRL group had a greater number of immature fibers than the Ble group. At 30 days, there was an absence of inflammation and equal proportion of mature and immature collagen fibers. All bleached groups showed a reduction in the volume of the pulp chamber.
Conclusion
Three consecutive applications of RL and one IRL application can minimize damage to the pulp of bleached teeth, whereas three IRL applications can minimize pulp fibrosis. However, LLLT did not prevent deposition of tertiary dentin.
Clinical relevance
This study describes LLLT protocols capable of minimizing inflammation and maturation of collagen fibers in pulp tissue after dental bleaching. However, the protocols proved insufficient for reducing the formation of tertiary dentin in bleached teeth.




Similar content being viewed by others
References
Korytowski W, Sarna T (1990) Bleaching of melanin pigments. Role of copper ions and hydrogen peroxide in autooxidation and photoxidation of synthetic dopa-melanin. J Biol Chem 265:12410–12416
Marson FC, Gonçalves RS, Silva CO, Cintra LT, Pascotto RC, Santos PH, Briso ALF (2015) Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper Dent 40:72–79
Cintra LTA, Benetti F, Ferreira LL, Gomes-Filho JE, Ervolino E, Gallinari MO, Rahal V, Briso ALF (2016) Penetration capacity, color alteration and biological response of two in-office bleaching protocols. Braz Dent J 27:169–175
Barbosa JG, Benetti F, de Oliveira Gallinari M, Carminatti M, da Silva ABD, Lopes INI, Briso ALF, Cintra LTA (2020) Bleaching gel mixed with MI Paste Plus reduces penetration of H2O2 and damage to pulp tissue and maintains bleaching effectiveness. Clin Oral Investig 24:1299–1309
Costa CAS, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:e59–e64
Roderjan DA, Stanislawczuk R, Hebling J, Costa CA, Reis A, Loguercio AD (2015) Response of human pulps to different in-office bleaching techniques: preliminary findings. Braz Dent J 26:242–248
Cintra LTA, Benetti F, Facundo ACS, Ferreira LL, Gomes-Filho JE, Ervolino E, Rahal V, Briso ALF (2013) The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 39:1576–1580
Benetti F, Gomes-Filho JE, Ferreira LL, Ervolino E, Briso ALF, Sivieri-Araújo G, Cintra LTA (2017) Hydrogen peroxide induces cell proliferation and apoptosis in pulp of rats after dental bleaching in vivo. Arch Oral Biol 81:103–109
Benetti F, Gomes-Filho JE, Ferreira LL, Sivieri-Araújo G, Ervolino E, Briso ALF, Cintra LTA (2018) Concentration-dependent effect of bleaching agents on the immunolabelling of interleukin-6, interleukin-17 and CD5-positive cells in the dental pulp. Int Endod J 51:789–799
Seale NS, McIntosh JE, Taylor AN (1981) Pulpal reaction to bleaching of teeth in dogs. J Dent Res 80:948–953
Cintra LTA, Benetti F, Ferreira LL, Rahal V, Ervolino E, Jacinto RC, Gomes Filho JE, Briso ALF (2016) Evaluation of an experimental rat model for comparative studies of bleaching agents. J Appl Oral Sci 24:95–104
Cintra LTA, Ferreira LL, Benetti F, Gastélum AA, Gomes-Filho JE, Ervolino E, Briso ALF (2017) The effect of dental bleaching on pulpal tissue response in a diabetic animal model. Int Endod J 50:790–798
Ferreira LL, Gomes-Filho JE, Benetti F, Carminatti M, Ervolino E, Briso ALF, Cintra LTA (2018) The effect of dental bleaching on pulpal tissue response in a diabetic animal model: a study of immunoregulatory cytokines. Int Endod J 51:347–356
Benetti F, Briso ALF, Carminatti M, de Araújo Lopes JM, Barbosa JG, Ervolino E, Gomes-Filho JE, Cintra LTA (2019) The presence of osteocalcin, osteopontin and reactive oxygen species-positive cells in pulp tissue after dental bleaching. Int Endod J 52:665–675
Benetti F, Briso ALF, de Araújo Lopes JM, Carminatti M, Conti LC, Gallinari MO, Ervolino E, Cintra LTA (2019) In vivo analysis of the presence of heme oxygenase-1, transcription factor Jun-D and CD90+/CD73+/CD105+/CD45- cells in the pulp of bleached teeth. Int Endod J 52:1723–1737
Faria-E-Silva AL, Nahsan FP, Fernandes MT, Martins-Filho PR (2015) Effect of preventive use of nonsteroidal anti-inflammatory drugs on sensitivity after dental bleaching: a systematic review and meta-analysis. J Am Dent Assoc 146:87–93
Rezende M, Loguercio AD, Kossatz S, Reis A (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: a multi regression and logistic analysis. J Dent 45:1–6
Charakorn P, Cabanilla LL, Wagner WC, Foong WC, Shaheen J, Pregitzer R, Schneider D (2009) The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. Oper Dent 34:131–135
Sasaki RT, Flório FM, Basting RT (2009) Effect of 10% sodium ascorbate and 10% alpha-tocopherol in different formulations on the shear bond strength of enamel and dentin submitted to a home-use bleaching treatment. Oper Dent 34:746–752
May LG, Salvia AC, Souza RO, Michida SM, Valera MC, Takahashi FE, Bottino MA (2010) Effect of sodium ascorbate and the time lapse before cementation after internal bleaching on bond strength between dentin and ceramic. J Prosthodont 19:374–380
Vargas FDAS, Soares DG, Basso FG, Hebling J (2014) Dose-response and time-course of α-tocoferol mediating the cytoprotection of dental pulp cells against hydrogen peroxide. Braz Dent J 25:367–371
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg 22:141–150
Silveira PCL, Streck EL, Pinho RA (2007) Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol B 86:279–282
Moosavi H, Arjmand N, Ahrari F, Zakeri M, Maleknejad F (2016) Effect of low-level laser therapy on tooth sensitivity induced by in-office bleaching. Lasers Med Sci 31:713–719
Dantas CM, Vivan CL, Ferreira LS, Freitas PM, Marques MM (2010) In vitro effect of low intensity laser on the cytotoxicity produced by substances released by bleaching gel. Braz Oral Res 24:460–466
Lima AF, Ribeiro AP, Basso FG, Bagnato VS, Hebling J, Marchi GM, de Souza Costa CA (2014) Effect of low-level laser therapy on odontoblast-like cells exposed to bleaching agent. Lasers Med Sci 29:1533–1538
Alencar CM, De Paula BLF, Araújo JLN, Alves EB, De Albuquerque Jassé FF, Silva CM (2018) Effect of low-level laser therapy combined with 5000 parts per million fluoride dentifrice on post bleaching sensitivity: a clinical, randomized, and double-blind study. J Esthet Restor Dent 30:352–359
Calheiros APC, Moreira MS, Gonçalves F, Aranha ACC, Cunha SR, Steiner-Oliveira C, Eduardo CP, Ramalho KM (2017) Photobiomodulation in the prevention of tooth sensitivity caused by in-office dental bleaching. A randomized placebo preliminary study. Photomed Laser Surg 35:415–420
de Paula B, Alencar C, Ortiz M, Couto R, Araújo J, Silva C (2019) Effect of photobiomodulation with low-level laser therapy combined with potassium nitrate on controlling post-bleaching tooth sensitivity: clinical, randomized, controlled, double-blind, and split-mouth study. Clin Oral Investig 23:2723–2732
Caviedes-Bucheli J, Ariza-García G, Restrepo-Méndez S, Ríos-Osorio N, Lombana N, Muñoz HR (2008) The effect of tooth bleaching on substance P expression in human dental pulp. J Endod 34:1462–1465
Benetti F, Lemos CAA, Oliveira Gallinari M, Terayama AM, Briso ALF, Castilho Jacinto R, Sivieri-Araújo G, Cintra LTA (2018) Influence of different types of light on the response of the pulp tissue in dental bleaching: a systematic review. Clin Oral Investig 22:1825–1837
Junqueira LC, Montes GS, Sanchez EM (1982) The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry 74:153–156
Martins CM, Sasaki H, Hirai K, Andrada AC, Gomes-Filho JE (2016) Relationship between hypertension and periapical lesion: an in vitro and in vivo study. Braz Oral Res 30:e78
Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S (2011) Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 26:1871–1882
Kang SY, Deshpande SS, Donneys A, Rodriguez JJ, Nelson NS, Felice PA, Chepeha DB, Buchman SR (2013) Parathyroid hormone reverses radiation induced hypovascularity in a murine model of distraction osteogenesis. Bone 56:9–15
Sun Z, Wang L, Peng B (2014) Kinetics of glycogen synthase kinase (GSK)3β and phosphorylated GSK3β (Ser 9) expression in experimentally induced periapical lesions. Int Endod J 47:1107–1116
Kalatzis-Sousa NG, Spin-Neto R, Wenzel A, Tanomaru-Filho M, Faria G (2017) Use of micro-computed tomography for the assessment of periapical lesions in small rodents: a systematic review. Int Endod J 50:352–366
Soares DG, Basso FG, Scheffel DS, Hebling J, de Souza Costa CA (2015) Responses of human dental pulp cells after application of a low-concentration bleaching gel to enamel. Arch Oral Biol 60:1428–1436
Louzada LM, Briso ALF, Benetti F, Vieira LB, Castilho Jacinto R, Dezan-Júnior E, Cintra LTA (2019) Anti-inflammatory potential of a carvedilol gel in the pulpal tissue of rats after dental bleaching: a histopathological evaluation. J Investig Clin Dent 10:e12401
Yu HS, Chang KL, Yu CL, Chen JW, Chen GS (1996) Low-energy helium-neon lasers irradiation stimulates interleukin-1 alpha and interleukin-8 release from cultured human keratinocytes. J Invest Dermatol 107:593–596
Lubart R, Friedmann H, Sinyakov M, Cohen N, Breibart H (1997) Changes in calcium transport in mammalian sperm motichondria and plasma membranes caused by 780 nm irradiation. Lasers Surg Med 21:493–499
Jori G, Schindl L, Schindl A, Polo N (1996) Novel approaches towards a detailed control of the mechanism and efficiency of photosensitized process in vivo. J Photochem Photobiol A 102:101–107
Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD (2005) Effect of wavelength on low-intensity laser irradiation- stimulated cell proliferation in vitro. Lasers Surg Med 36:8–12
Hawkins V, Abrahamse H (2006) Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24:705–714
Buchalla W, Attin T (2007) External bleaching therapy with activation by heat, light or laser-a systematic review. Dent Mater 23:586–596
Yamakawa S, Niwa T, Karakida T, Kobayashi K, Yamamoto R, Chiba R, Yamakoshi Y, Hosoya N (2018) Effects of Er:YAG and diode laser irradiation on dental pulp cells and tissues. Int J Mol Sci 19:E2429
Abrahão IJ, Martins MD, Katayama E, Antoniazzi JH, Segmentilli A, Marques MM (2006) Collagen analysis in human tooth germ papillae. Braz Dent J 17:208–212
Benetti F, Briso ALF, Ferreira LL, Carminatti M, Álamo L, Ervolino E, Dezan-Júnior E, Cintra LTA (2018) In vivo study of the action of a topical anti-inflammatory drug in rat teeth submitted to dental bleaching. Braz Dent J 29:555–561
Dong J, Ma Q (2017) Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation. Part Fibre Toxicol 14:18
Garrido PR, Pedroni ACF, Cury DP, Moreira MS, Rosin F, Sarra G, Marques MM (2019) Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J Photochem Photobiol B 94:149–157
Pyo SJ, Song WW, Kim IR, Park BS, Kim CH, Shin SH, Chung IK, Kim YD (2013) Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med Sci 28:543–550
de Oliveira LSS, de Araújo AA, de Araújo Júnior RF, Barboza CAG, Borges BCD, da Silva JSP (2017) Low-level laser therapy (780 nm) combined with collagen sponge scaffold promotes repair of rat cranial critical-size defects and increases TGF-β, FGF-2, OPG/RANK and osteocalcin expression. Int J Exp Pathol 98:75–85
Pinheiro CCG, de Pinho MC, Aranha AC, Fregnani E, Bueno DF (2018) Low power laser therapy: a strategy to promote the osteogenic differentiation of deciduous dental pulp stem cells from cleft lip and palate patients. Tissue Eng Part A 24:569–575
Hebling J, Giro EMA, Costa CAS (1999) Biocompatibility of an adhesive system applied to exposed human dental pulp. J Endod 25:676–682
Costa CA, Oliveira MF, Giro EM, Hebling J (2003) Biocompatibility of resin-based materials used as pulp-capping agents. In Endod J 36:831–839
Dammaschke T (2010) Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim 44:1–6
Sasaki T, Kawamata-Kido H (1995) Providing an environment for reparative dentine induction in amputated rat molar pulp by high molecular-weight hyaluronic acid. Arch Oral Biol 40:209–219
Hunt HR, Rosen S, Hoppert CA (1970) Morphology of molar teeth and occlusion in young rats. J Dent Res 49:508–514
Funding
This study was supported by a grant (2016/20271-7) from the Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo, SP, Brazil and by a grant (311650/2018-0) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, São Paulo, SP, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. The in vivo (CEUA 00938) study using rat molars was approved by the Ethics Committee of the institution in which the study was performed and conducted in accordance with its ethical standards.
Informed consent
For this type of study, informed consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Terayama, A.M., Benetti, F., de Araújo Lopes, J.M. et al. Influence of low-level laser therapy on inflammation, collagen fiber maturation, and tertiary dentin deposition in the pulp of bleached teeth. Clin Oral Invest 24, 3911–3921 (2020). https://doi.org/10.1007/s00784-020-03258-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03258-9