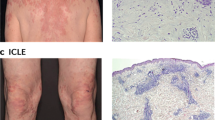
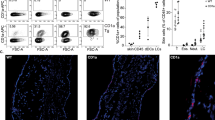
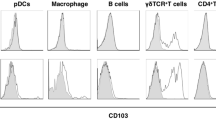
Abstract
Innate lymphoid cells (ILCs), as an important component of the innate immune system, arise from a common lymphoid progenitor and are located in mucosal barriers and various tissues, including the intestine, skin, lung, and adipose tissue. ILCs are heterogeneous subsets of lymphocytes that have emerging roles in orchestrating immune response and contribute to maintain metabolic homeostasis and regulate tissue inflammation. Currently, more details about the pathways for the development and differentiation of ILCs have largely been elucidated, and cytokine secretion and downstream immune cell responses in disease pathogenesis have been reported. Recent research has identified that several distinct subsets of ILCs at skin barriers are involved in the complex regulatory network in local immunity, potentiating adaptive immunity and the inflammatory response. Of note, additional studies that assess the effects of ILCs are required to better define how ILCs regulate their development and functions and how they interact with other immune cells in autoimmune-related and inflammatory skin disorders. In this review, we will distill recent research progress in ILC biology, abnormal functions and potential pathogenic mechanisms in autoimmune-related skin diseases, including systemic lupus erythematosus (SLE), scleroderma and inflammatory diseases, as well as psoriasis and atopic dermatitis (AD), thereby giving a comprehensive review of the diversity and plasticity of ILCs and their unique functions in disease conditions with the aim to provide new insights into molecular diagnosis and suggest potential value in immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Minton, K. Innate lymphoid cells: ILC diversity maintained by microbiota. Nat. Rev. Immunol. 16, 593 (2016).
Klose, C. S. & Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 17, 765–774 (2016).
Tait, W. E. & Artis, D. Innate lymphoid cells: Balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe 12, 445–457 (2012).
Tugues, S. et al. Innate lymphoid cells as regulators of the tumor microenvironment. Semin. Immunol. 41, 101270 (2019).
Spits, H. & Cupedo, T. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30, 647–675 (2012).
Powell, N. et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 37, 674–684 (2012).
Weizman, O. E. et al. ILC1 confer early host protection at initial sites of viral infection. Cell 171, 795–808 (2017).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Wilhelm, C. et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12, 1071–1077 (2011).
Monticelli, L. A. et al. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc. Natl Acad. Sci. USA 112, 10762–10767 (2015).
Yasuda, K. et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl Acad. Sci. USA 109, 3451–3456 (2012).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Moro, K. et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Hoyler, T. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012).
Wong, S. H. et al. Transcription factor RORalpha is critical for nuocyte development. Nat. Immunol. 13, 229–236 (2012).
Teunissen, M. et al. Composition of innate lymphoid cell subsets in the human skin: Enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J. Invest. Dermatol. 134, 2351–2360 (2014).
Ciccia, F. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747 (2015).
Montaldo, E. et al. Human RORgammat(+)CD34(+) cells are lineage-specified progenitors of group 3 RORgammat(+) innate lymphoid cells. Immunity 41, 988–1000 (2014).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Tlaskalova-Hogenova, H. et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 93, 97–108 (2004).
Hou, M. & Liu, S. Innate lymphoid cells are increased in systemic lupus erythematosus. Clin. Exp. Rheumatol. 37, 676–679 (2019).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573 (2013).
Spits, H. et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–1246 (2016).
Kiessling, R. et al. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 5, 112–117 (1975).
Mebius, R. E. et al. Developing lymph nodes collect CD4+ CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Klose, C. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Carotta, S. et al. Identification of the earliest NK-cell precursor in the mouse BM. J. Exp. Med. 203, 1105–1116 (2011).
Constantinides, M. G. et al. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Rawlins, E. L. et al. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136, 3741–3745 (2009).
Boos, M. D. et al. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130 (2007).
Daussy, C. et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 211, 563–577 (2014).
Mjosberg, J. et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 37, 649–659 (2012).
Luci, C. et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Hudspeth, K. et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 66, 40–50 (2016).
Male, V. et al. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur. J. Immunol. 41, 3017–3027 (2011).
Marquardt, N. et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(-)CD56(dim) cells. J. Allergy Clin. Immunol. 139, 1321–1330 (2017).
Beziat, V. et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 121, 2678–2688 (2013).
Li, F. et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology 144, 392–401 (2013).
Schlums, H. et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42, 443–456 (2015).
Krneta, T. et al. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell. Mol. Immunol. 13, 628–639 (2016).
Zheng, X. et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 20, 1656–1667 (2019).
Davies, A. J. et al. Natural killer cells degenerate intact sensory afferents following nerve injury. Cell 176, 716–728 (2019).
Aguilar, O. A. et al. A viral immunoevasin controls innate immunity by targeting the prototypical natural killer cell receptor family. Cell 169, 58–71 (2017).
Bottcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018).
Gauthier, L. et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 177, 1701–1713 (2019).
Anft, M. et al. NK cell detachment from target cells is regulated by successful cytotoxicity and influences cytokine production. Cell. Mol. Immunol. https://doi.org/10.1038/s41423-019-0277-2 (2019).
Bernink, J. H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Jiang, X. et al. Single line or parallel lines: NK cell differentiation driven by T-bet and Eomes. Cell. Mol. Immunol. 9, 193–194 (2012).
Constantinides, M. G. et al. PLZF expression maps the early stages of ILC1 lineage development. Proc. Natl Acad. Sci. USA 112, 5123–5128 (2015).
Wang, X. et al. Memory formation and long-term maintenance of IL-7Ralpha(+) ILC1s via a lymph node-liver axis. Nat. Commun. 9, 4854 (2018).
Fort, M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity (2010).
Guo, L. et al. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 33, 598–606 (2012).
Doherty, T. A. et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 132, 205–213 (2013).
Ohne, Y. et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol. 17, 646–655 (2016).
Noval, R. M. et al. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 138, 801–811 (2016).
Bal, S. M. et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 17, 636–645 (2016).
Tan, Z. et al. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell. Mol. Immunol. 15, 388–398 (2018).
von Moltke, J. et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Schneider, C. et al. A Metabolite-Triggered tuft Cell-ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284 (2018).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Moriyama, S. et al. Beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Kurebayashi, S. et al. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl Acad. Sci. USA 97, 10132–10137 (2000).
Cupedo, T. et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC + CD127+ natural killer-like cells. Nat. Immunol. 10, 66–74 (2009).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Wang, Q. et al. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol. 4, eaay7501, https://doi.org/10.1126/sciimmunol.aay7501 (2019).
Bernink, J. H. et al. C-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 20, 992–1003 (2019).
Stockinger, B. et al. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin. Immunol. 23, 99–105 (2011).
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Li, S. et al. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity 49, 915–928 (2018).
Carrega, P. et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 6, 8280 (2015).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Abou-Samra, E. et al. NKR-P1B expression in gut-associated innate lymphoid cells is required for the control of gastrointestinal tract infections. Cell. Mol. Immunol. 16, 868–877 (2019).
Ibiza, S. et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443 (2016).
Godinho-Silva, C. et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature 574, 254–258 (2019).
Dalli, J. et al. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity 46, 92–105 (2017).
Hepworth, M. R. et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science 348, 1031–1035 (2015).
Orlik, C. et al. Keratinocytes costimulate naive human T cells via CD2: A potential target to prevent the development of proinflammatory Th1 cells in the skin. Cell. Mol. Immunol. https://doi.org/10.1038/s41423-019-0261-x (2019).
Nestle, F. O. et al. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Kabashima, K. et al. The immunological anatomy of the skin. Nat. Rev. Immunol. 19, 19–30 (2019).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192 (2009).
Gallo, R. L. Human skin is the largest epithelial surface for interaction with microbes. J. Invest. Dermatol. 137, 1213–1214 (2017).
Kobayashi, T. et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 176, 982–997 (2019).
Schwartz, C. et al. Spontaneous atopic dermatitis in mice with a defective skin barrier is independent of ILC2 and mediated by IL-1beta. Allergy 74, 1920–1933 (2019).
Koues, O. I. et al. Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell 165, 1134–1146 (2016).
Tsoi, L. C. et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 44, 1341–1348 (2012).
Ebihara, T. et al. Runx3?specifies lineage commitment of innate lymphoid cells. Nat. Immunol. 16, 1124–1133 (2015).
Murthy, A. et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 36, 105–119 (2012).
Demehri, S. et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 6, e123 (2008).
Siracusa, M. C. et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477, 229–233 (2011).
Cherrier, M. et al. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J. Exp. Med. 209, 729–740 (2012).
Zhang, K. et al. Cutting edge: Notch signaling promotes the plasticity of group-2 innate lymphoid cells. J. Immunol. 198, 1798–1803 (2017).
D’Cruz, D. P. et al. Systemic lupus erythematosus. Lancet 369, 587–596 (2007).
Dorner, T. & Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 393, 2344–2358 (2019).
Chiche, L. et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 66, 1583–1595 (2014).
Liu, M. et al. Type I interferons promote the survival and proinflammatory properties of transitional B cells in systemic lupus erythematosus patients. Cell. Mol. Immunol. 16, 367–379 (2019).
Chang, N. H. et al. Interferon-alpha induces altered transitional B cell signaling and function in Systemic Lupus Erythematosus. J. Autoimmun. 58, 100–110 (2015).
Duster, M. et al. T cell-derived IFN-gamma downregulates protective group 2 innate lymphoid cells in murine lupus erythematosus. Eur. J. Immunol. 48, 1364–1375 (2018).
Guo, C. et al. Innate lymphoid cell disturbance with increase in ILC1 in systemic lupus erythematosus. Clin. Immunol. 202, 49–58 (2019).
Blokland, S. et al. Increased expression of Fas on group 2 and 3 innate lymphoid cells is associated with an interferon signature in systemic lupus erythematosus and Sjogren’s syndrome. Rheumatol. (Oxf.) 58, 1740–1745 (2019).
Schepis, D. et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology 126, 140–146 (2009).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Lowes, M. A. et al. Immunology of psoriasis. Annu. Rev. Immunol. 32, 227–255 (2014).
Nair, R. P. et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 78, 827–851 (2006).
Tervaert, W. C. & Esseveld, H. A study of the incidence of haemolytic streptococci in the throat in patients with psoriasis vulgaris, with reference to their role in the pathogenesis of this disease. Dermatologica 140, 282–290 (1970).
Thorarensen, S. M. et al. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 76, 521–525 (2017).
Brauchli, Y. B. et al. Association between beta-blockers, other antihypertensive drugs and psoriasis: Population-based case-control study. Br. J. Dermatol. 158, 1299–1307 (2008).
Becher, B. & Pantelyushin, S. Hiding under the skin: interleukin-17-producing gammadelta T cells go under the skin? Nat. Med 18, 1748–1750 (2012).
Pantelyushin, S. et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 (2012).
Ivanov, I. I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Villanova, F. et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Invest. Dermatol. 134, 984–991 (2014).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Bruggen, M. C. et al. In situ mapping of innate lymphoid cells in human skin: Evidence for remarkable differences between normal and inflamed skin. J. Invest. Dermatol. 136, 2396–2405 (2016).
Dyring-Andersen, B. et al. Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. Br. J. Dermatol. 170, 609–616 (2014).
Paternoster, L. et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 47, 1449–1456 (2015).
Palmer, C. N. et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 38, 441–446 (2006).
Rodriguez, E. et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. J. Allergy Clin. Immunol. 123, 1361–1370 (2009).
Kim, B. S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 116r–170r (2013).
Saunders, S. P. et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 137, 482–491 (2016).
Chen, L. Y. et al. Protein palmitoylation by ZDHHC13 protects skin against microbial-driven dermatitis. J. Invest. Dermatol. 137, 894–904 (2017).
Li, Y. et al. Kinetics of the accumulation of group 2 innate lymphoid cells in IL-33-induced and IL-25-induced murine models of asthma: a potential role for the chemokine CXCL16. Cell. Mol. Immunol. 16, 75–86 (2019).
Barlow, J. L. et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 132, 933–941 (2013).
Salimi, M. et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 210, 2939–2950 (2013).
Xue, L. et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 133, 1184–1194 (2014).
Kim, B. S. et al. Basophils promote innate lymphoid cell responses in inflamed skin. J. Immunol. 193, 3717–3725 (2014).
Mashiko, S. et al. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J. Dermatol. Sci. 88, 167–174 (2017).
Jiao, D. et al. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell. Mol. Immunol. 13, 535–550 (2016).
Irani, A. M. et al. Immunohistochemical detection of human basophils in late-phase skin reactions. J. Allergy Clin. Immunol. 101, 354–362 (1998).
Denton, C. P. & Khanna, D. Systemic sclerosis. Lancet. 390, 1685–1699 (2017).
Foocharoen, C. et al. Clinical characteristics of scleroderma overlap syndromes: Comparisons with pure scleroderma. Int. J. Rheum. Dis. 19, 913–923 (2016).
Rezaei, R. et al. Genetic implications in the pathogenesis of systemic sclerosis. Int. J. Rheum. Dis. 21, 1478–1486 (2018).
De Martinis, M. et al. An overview of environmental risk factors in systemic sclerosis. Expert Rev. Clin. Immunol. 12, 465–478 (2016).
Airo’, P. et al. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. J. Rheumatol. 38, 1329–1334 (2011).
Joseph, C. G. et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014).
Roan, F. et al. CD4+ group 1 innate lymphoid cells (ILC) form a functionally distinct ILC subset that is increased in systemic sclerosis. J. Immunol. 196, 2051–2062 (2016).
Aliprantis, A. O. et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc. Natl Acad. Sci. USA 104, 2827–2830 (2007).
Wohlfahrt, T. et al. Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann. Rheum. Dis. 75, 623–626 (2016).
Cohen, T. J. & Damoiseaux, J. Antineutrophil cytoplasmic autoantibodies: How are they detected and what is their use for diagnosis, classification and follow-up? Clin. Rev. Allergy Immunol. 43, 211–219 (2012).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Sunderkotter, C. H. et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. 70, 171–184 (2018).
Braudeau, C. et al. Persistent deficiency of circulating mucosal-associated invariant T (MAIT) cells in ANCA-associated vasculitis. J. Autoimmun. 70, 73–79 (2016).
Mowad, C. M. Patch testing: pitfalls and performance. Curr. Opin. Allergy Clin. Immunol. 6, 340–344 (2006).
Kaplan, D. H. et al. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 12, 114–124 (2012).
Rafei-Shamsabadi, D. A. et al. Lack of type 2 innate lymphoid cells promotes a type I-Driven enhanced immune response in contact hypersensitivity. J. Invest. Dermatol. 138, 1962–1972 (2018).
Keren, A. et al. Innate lymphoid cells 3 induce psoriasis in xenotransplanted healthy human skin. J. Allergy Clin. Immunol. 142, 305–308 (2018).
Van Belle, A. B. et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 188, 462–469 (2012).
Imura, C. et al. A novel RORgammat inhibitor is a potential therapeutic agent for the topical treatment of psoriasis with low risk of thymic aberrations. J. Dermatol. Sci. 93, 176–185 (2019).
Rompoti, N. et al. Real world data from a single Greek center on the use of secukinumab in plaque psoriasis: effectiveness, safety, drug survival, and identification of patients that sustain optimal response. J. Eur. Acad. Dermatol. Venereol. (2020).
Merola, J. F. et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. J. Eur. Acad. Dermatol. Venereol. https://doi.org/10.1111/jdv.16181 (2020).
Bilal, J. et al. A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J. Dermatolog. Treat. 29, 569–578 (2018).
Dai, J. et al. Topical ROR inverse agonists suppress inflammation in mouse models of atopic dermatitis and acute irritant dermatitis. J. Invest. Dermatol. 137, 2523–2531 (2017).
Imai, Y. et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl Acad. Sci. USA 110, 13921–13926 (2013).
Oldhoff, J. M. et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 60, 693–696 (2005).
Pavord, I. D. et al. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet. 380, 651–659 (2012).
Ortega, H. G. et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 371, 1198–1207 (2014).
Mora-Velandia, L. M. et al. A human lin(-) CD123(+) CD127(low) population endowed with ILC features and migratory capabilities contributes to immunopathological hallmarks of psoriasis. Front Immunol. 8, 176 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81972943, No.81830097) and Hunan Talent Young Investigator (No. 2019RS2012).
Author information
Authors and Affiliations
Contributions
S.Q.Z. wrote the manuscript, Q.W.L. did the editing, H.J.W. and Q.J.L. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhou, S., Li, Q., Wu, H. et al. The pathogenic role of innate lymphoid cells in autoimmune-related and inflammatory skin diseases. Cell Mol Immunol 17, 335–346 (2020). https://doi.org/10.1038/s41423-020-0399-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-0399-6
Keywords
This article is cited by
-
Dermis resident macrophages orchestrate localized ILC2 eosinophil circuitries to promote non-healing cutaneous leishmaniasis
Nature Communications (2023)
-
Immune cell dysregulation as a mediator of fibrosis in systemic sclerosis
Nature Reviews Rheumatology (2022)