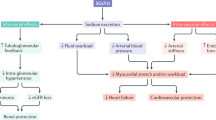
Abstract
Acute decline in estimated glomerular filtration rate (eGFR), a typical finding after initiating sodium-glucose cotransporter 2 (SGLT2) inhibitors, is associated with maintaining renal function in type 2 diabetes. However, the relationship between the magnitude of acute decline in eGFR and the course of eGFR thereafter is not known. A pooled analysis of four 52-week phase III trials of luseogliflozin 2.5 mg daily (or up to 5 mg daily) in Japanese patients with type 2 diabetes was conducted and stratified according to the tertile of magnitude of acute change in eGFR during the 2 weeks after initiation. The mean age, glycated hemoglobin, eGFR, and urinary albumin were 60 years, 7.8%, 79.6 mL/min/1.73 m2, and 62.7 mg/g Cr, respectively. Acute change in eGFR varied widely between patients (N = 941; mean, −2.3; min, −35.5; max, 27.6). Patients with greater acute decline in eGFR, characterized by higher baseline eGFR and increased diuretic use, showed rapid recovery and maintenance of eGFR thereafter. Higher eGFR, longer duration of diabetes, and higher body mass index and diuretic use were associated with greater acute decline in eGFR. The course of eGFR from 12 to 52 weeks was maintained regardless of acute changes. Although acute changes in eGFR varied widely among patients with type 2 diabetes, the course of eGFR thereafter was stable regardless of the degree of acute changes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Heise T, Seewaldt-Becker E, Macha S, Hantel S, Pinnetti S, Seman L, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613–21.
Katayama S, Hatano M, Issiki M. Clinical features and therapeutic perspectives on hypertension in diabetics. Hypertens Res. 2018;41:213–29.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72.
Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–76.
Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–83.
Vestri S, Okamoto MM, de Freitas HS, Aparecida Dos Santos R, Nunes MT, Morimatsu M, et al. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol. 2001;182:105–12.
Bank N, Aynedjian HS. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Investig. 1990;86:309–16.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–7.
Seino Y, Inagaki N, Haneda M, Kaku K, Sasaki T, Fukatsu A, et al. Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2015;6:443–53.
Seino Y, Kaku K, Inagaki N, Haneda M, Sasaki T, Fukatsu A, et al. Fifty-two-week long-term clinical study of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Endocr J. 2015;62:593–603.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, et al. Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40.
Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30:1735–45.
Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97.
Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140:303–15.
Zingerman B, Herman-Edelstein M, Erman A, Bar Sheshet Itach S, Ori Y, Rozen-Zvi B, et al. Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS ONE. 2015;10:e0137163.
Morales E, Valero MA, León M, Hernández E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41:319–27.
Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, et al. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension. 2016;68:1355–64.
Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798–810.
Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21:1291–8.
Takiyama Y, Sera T, Nakamura M, Ishizeki K, Saijo Y, Yanagimachi T, et al. Impacts of diabetes and an SGLT2 inhibitor on the glomerular number and volume in db/db mice, as estimated by synchrotron radiation micro-CT at SPring-8. EBioMedicine. 2018;36:329–46.
Hill GS, Heudes D, Bariéty J. Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int. 2003;63:1027–36.
Yasui A, Lee G, Hirase T, Kaneko T, Kaspers S, von Eynatten M, et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018;9:863–71.
Perkovic V, Jardine M, Vijapurkar U, Meininger G. Renal effects of canagliflozin in type 2 diabetes mellitus. Curr Med Res Opin. 2015;31:2219–31.
Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, et al. EMPA-REG OUTCOME Investigators. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018;29:2755–69.
Acknowledgements
This study was funded by Taisho Pharmaceutical Co., Ltd. Statistical analysis of this study was conducted at Taisho Pharmaceutical Co., Ltd. Editorial assistance for the preparation of this paper was provided by Cactus Communications and funded by Taisho Pharmaceutical Co., Ltd. We thank Dr Haneda, Dr Inagaki, Dr Kaku, Dr Sasaki, Dr Fukatsu, and all other physicians who participated in the phase III trial of luseogliflozin.
Author information
Authors and Affiliations
Contributions
KK wrote the paper and researched the data. YO contributed to the discussion. YS reviewed the paper. HY and HT researched the data and contributed to the discussion.
Corresponding author
Ethics declarations
Conflict of interest
KK has received speaker’s fees from Nippon Boehringer Ingelheim Co., Ltd, Mitsubishi Tanabe Pharma Co., Ltd, Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd, AstraZeneca K.K., and Taisho Toyama Pharmaceutical Co., Ltd. YO has received speaker’s fees from Nippon Boehringer Ingelheim Co., Ltd, Takeda Pharmaceutical Co., Ltd, Daiichi Sankyo Pharmaceutical Co., Ltd. YS has received consulting and/or speaker’s fees from MSD K.K., Kao Co., Ltd, Novo Nordisk Pharma Inc., Taisho Pharmaceutical Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd, Nippon Becton, Dickinson and Company, Nippon Boehringer Ingelheim Co., Ltd, and Takeda Pharmaceutical Co., Ltd. HY and HT are employees of Taisho Pharmaceutical Co., Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kohagura, K., Yamasaki, H., Takano, H. et al. Luseogliflozin, a sodium-glucose cotransporter 2 inhibitor, preserves renal function irrespective of acute changes in the estimated glomerular filtration rate in Japanese patients with type 2 diabetes. Hypertens Res 43, 876–883 (2020). https://doi.org/10.1038/s41440-020-0426-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0426-0
Keywords
This article is cited by
-
A systematic review of sodium-glucose cotransporter 2 inhibitors and renal profiles among Japanese patients with type 2 diabetes mellitus
Journal of Pharmaceutical Health Care and Sciences (2023)
-
Beneficial effects of luseogliflozin on lipid profile and liver function in patients with type 2 diabetes mellitus (BLUE trial): a single-center, single-arm, open-label prospective study
Diabetology & Metabolic Syndrome (2023)
-
Canagliflozin independently reduced plasma volume from conventional diuretics in patients with type 2 diabetes and chronic heart failure: a subanalysis of the CANDLE trial
Hypertension Research (2023)
-
Effects of SGLT2 inhibitors on eGFR in type 2 diabetic patients—the role of antidiabetic and antihypertensive medications
Hypertension Research (2021)