Abstract
As diets change in response to ethical, environmental, and health concerns surrounding meat consumption, fermentation has potential to improve the taste and nutritional qualities of plant-based foods. In this study, cauliflower, white beans, and a 50:50 cauliflower-white bean mixture were fermented using different strains of Lactobacillus plantarum. In all treatments containing cauliflower, the pH was reduced to <4 after 18 h, while treatments containing only white beans had an average pH of 4.8 after 18 h. Following fermentation, the riboflavin, folate, and vitamin B12 content of the cauliflower-white bean mixture was measured, and compared against that of an unfermented control. The riboflavin and folate content of the mixture increased significantly after fermentation. Relative to control samples, riboflavin increased by 76–113%, to 91.6 ± 0.6 μg/100 g fresh weight, and folate increased by 32–60%, to 58.8 ± 2.0 μg/100 g fresh weight. For one bacterial strain, L. plantarum 299, a significant 66% increase in vitamin B12 was observed, although the final amount (0.048 ± 0.013 μg/100 g fresh weight) was only a small fraction of recommended daily intake. Measurements of amino acid composition in the mixture revealed small increases in alanine, glycine, histidine, isoleucine, leucine, and valine in the fermented sample compared to the unfermented control.
Similar content being viewed by others
Introduction
Recent research has highlighted good potential of a change in diet in helping to resolve global challenges such as climate change, biodiversity loss, and food insecurity [1]. Studies exploring future sustainable food systems in the Nordic countries suggest decreasing consumption of meat by 80–90% and increasing consumption of vegetables. Legumes, with their high protein content, are of special importance in this concern due to their benefits for agricultural cropping systems via biological nitrogen-fixation [2]. Thus, there is a need for developing new plant-based products including legumes.
Fermentation has been used since ancient times for food preservation, while also having an impact on organoleptic characteristics. Several traditional Asian fermented bean products have now become popular in the West, including tofu, tempeh, and miso. Additional driving forces in developing fermented vegetable products are the growing interest in locally produced food, and consumer interest in products with less chemical additives.
During fermentation with lactic acid bacteria (LAB), available carbohydrates are converted to organic acids, mainly lactic acid and acetic acid, depending on the species used. For vegetables, a decrease in pH to around 4 has been reported to ensure a stable product [3]. The dominant species in spontaneous lactic acid fermentation of vegetables is Lactobacillus plantarum [4]. A benefit of using well-known lactic acid bacteria, such as L. plantarum, for fermentation is that they are included in the Qualified Presumption of Safety (QPS) list, which authorizes their use in the food and feed chain within the European Union.
The aim in lactic acid fermentation is generally to preserve the food by excluding growth of spoilage microorganisms. However, lactic acid fermentation is a strain-dependent and complex process with a broad impact on the nutritional value of the food [5]. An increase in the content of important nutrients, including the B-vitamins, after fermentation of plant-based products has been reported [6]. Apart from the direct effect on the food due to the bacterial metabolism, certain strains of LAB are also associated with probiotic properties. The L. plantarum strains investigated in the present study have been shown to have different health effects in humans, for example improved symptoms in people with irritable bowel syndrome [7], protection against lumbar spine bone loss in postmenopausal women [8], and increased iron absorption from foods [9].
The growth and capability for efficient fermentation of LAB are affected by several factors, such as composition of the substrate, strain-specific variations, and the fermentation procedure. In the present study fermentation of vegetables, cauliflower, white beans, and a mixture (50:50) of cauliflower and white beans, was studied. Four strains of L. plantarum were used and the effects of fermentation on levels of important nutritional parameters such as amino acid composition and riboflavin, folate, and vitamin B12 content were studied. Three of the investigated strains are available commercially as food supplements and as chilled plant-based food products and have been used to ferment cereals, berries and fruit [10,11,12]. The ability of the included strains to ferment vegetables and produce riboflavin, folate and vitamin B12 have not been investigated before.
Material and Methods
Bacterial Cultures
Four different strains of L. plantarum (strain 299v, strain Lp900, strain 299, strain Heal19) were provided by the company Probi AB, Sweden (https://probi.com/) and are described in Table 1. For production of the inoculum used for fermentation, the strains were cultivated as static culture in MRS broth (BDH Chemicals, UK) at 35 °C for 16 h. After this, the cells were harvested by centrifugation (Eppendorf MiniSpin, 10,000 g for 3 min) and washed twice with 0.85% NaCl solution. The control treatment was prepared with sterile MRS broth and a similar washing procedure. The bacterial suspensions, diluted in 0.85% NaCl and with an OD620 of 0.8 (corresponding to 7–8 log CFU/ml), and a control suspension (0.85% NaCl only) were added in a concentration of 1% (w/w) to the vegetable mixtures.
Experimental Set-up
Raw cauliflower (Brassica oleracea var. botrytis) mixed in food processor, cooked and mixed white beans (Phaseolus vulgaris L.), and a mixture of consisting of a 50:50 ratio (w/w) of raw cauliflower and cooked white beans (cauliflower-white bean) were weighed out into plastic containers. Each portion weighed 100 g ± 1 g and was combined with 2 g of sea salt.
Suspension of L. plantarum was added to each container. The mixture was thoroughly stirred again following addition of bacteria or control suspension. The pH of each sample was recorded and the control samples were directly frozen at −80 °C. Containers with bacterial culture were incubated at 30 °C for 44 h. The pH of fermented samples was measured after 18 and 44 h.
After 44 h, the treatments were tasted and the cauliflower-white bean mixture was chosen for further analysis. Samples for determination of riboflavin (vitamin B2), folate, vitamin B12, total protein, and amino acid composition in this treatment were prepared and frozen at −80 °C. All samples were analyzed for total protein and vitamin content, while analyses of amino acid composition were performed on the control samples of cauliflower-white bean mixture and the samples fermented with L. plantarum 299.
Analysis
Determination of Riboflavin, Total Amount of Folate and Vitamin B12
Determination of riboflavin was performed according to European Standard EN14152, as described by Jakobsen [13]. Determination of the total amount of folate was performed according to European Standard EN1413, as described by DeVries et al. [14], except for use of protease in the extraction procedure. Extraction of vitamin B12 was performed according to method AOAC 952.20, as described by Ball [15].
Total Protein and Amino Acid Composition
Total amount of protein in the samples was determined by the Dumas method [16] and applying a conversion factor of 6.25 for total nitrogen. Concentrations of the amino acids were determined according to the method of Llames and Fontaine [17].
Statistics
The experiments were set up with three replicates in each treatment and repeated once. The data obtained were analyzed statistically using Minitab 17 for Windows. One-way Anova followed by Tukey’s multiple comparison test was employed to test for effects of treatments and the significance level was set to P ≤ 0.05.
Results and Discussion
The different strains of L. plantarum behaved similarly with regard to the effect of pH on the different treatments. No significant difference was observed between the strains within each reading (18 and 44 h). Data from all strains were therefore pooled for each time point for analysis of the effect of fermentation on pH (Fig. 1). Cauliflower and white bean had a similar initial pH of approximately 6.20. However, after 18 h of fermentation with L. plantarum strains, the pH was significantly lower in the cauliflower and the cauliflower-white bean mixture treatments (3.66 ± 0.05 and 3.75 ± 0.04, respectively, mean ± SD) than in the treatment with white bean only (4.82 ± 0.02). After 44 h of fermentation, a slight but significant increase in pH was observed in the white bean treatment, to 4.96 ± 0.01. In the treatments with cauliflower and cauliflower/white bean mixture the opposite pattern was observed, with a slight but significant decrease in pH to 3.44 ± 0.1 and 3.52 ± 0.07, respectively.
Thus, fermentation was more efficient in the treatments including cauliflower adding benefits such as increased shelf life due to the low pH. Cauliflower is reported to contain approximately 4.2% carbohydrates, with a high concentration of monosaccharides [18]. Legumes, on the other hand, are well-known for containing high amounts of complex oligosaccharides, a component of dietary fiber that is less available for microbial degradation [19]. Thus, the easily available carbohydrates provided by cauliflower most probably sustained microbial growth, followed by a decrease in pH due to production of organic acids, to a greater extent than in the white bean treatment.
The mixture of cauliflower and white bean was chosen for further studies on the content of total protein, riboflavin, folate, and vitamin B12. The taste was dominated by a sour and salty flavour, similar to traditional fermented cabbage (sauerkraut), but with a deeper, underlying umami taste that brought a mild cheese-like quality. The taste was unusual, but not unpleasant, though more comprehensive taste analysis and consumer research would be required to determine the marketability of the product.
From a nutritional perspective the benefit of including white beans was clearly apparent, as the total amount of protein ranged from 21.1 to 23.2% of dry weight in the different bacterial treatments. Inconsistent results have been reported regarding the effect of fermentation on total protein amount [20]. Based on work with cereals, it has been suggested that the total amount of protein is generally not changed during lactic acid fermentation, although an increase can be observed in certain cases. If an increase is observed, it can often be related to a decrease in carbon ratio in the total mass due to bacterial metabolization of carbohydrates [21]. In the present study, no significant differences were observed in organic carbon content or in total nitrogen in relation to the control or between any of the treatments. Based on these results, no effect on the total protein amount was observed.
Riboflavin is important for the function of several enzymes involved in energy metabolism. It is naturally present in several different foods, including plants, and main sources of riboflavin intake are milk and dairy products, followed by cereals and meat. It is a water-soluble vitamin that is not stored in the body, and the daily dietary reference value has been set to 1.6 mg for adults [22]. Despite its presence in a wide variety of foods, riboflavin deficiency may occur. In this study, fermentation with L. plantarum increased the concentration of riboflavin significantly compared to the control in all treatments (Table 2). Significant differences in riboflavin concentration related to the different strains were observed. The highest value was observed after fermentation with L. plantarum Lp900, which gave an increase of 113% compared to the initial value, to 91.6 ± 0.6 μg/100 g fresh weight. The smallest increase, 76% of the initial value, was observed in the treatment with L. plantarum 299v. A similar increase in riboflavin content has been observed by Capozzi et al. [23] on fermenting wheat with L. plantarum for production of bread and pasta. However, in their study the strains used were selected for over-production of riboflavin, while such selection was not applied in the present study. Our results suggest that fermentation with L. plantarum can be used to increase the concentration of riboflavin in plant-based foods. However, it should be pointed out that the levels of riboflavin in the final fermented product were still generally low, at the level of μg/100 g product, compared to the recommended daily intake of 1.6 mg.
For folate, a similar pattern as for riboflavin was observed, with a significant increase in all fermented samples and variations between strains. Like riboflavin, folate is synthesized by both plants and microorganisms, with main dietary sources being leafy green vegetables, dairy products, and cereal products. This vitamin, including several related compounds play a key role in ensuring essential functions of cell metabolism, such as DNA synthesis. However, the bioavailability of natural food folates varies and these compounds are easily degraded. Thus, folate deficiency is a general concern, and a strategy based on fortification of selected foods has been adopted in some countries. In this study, the highest concentration of folate was observed after fermentation with L. plantarum 299v, which showed an increase of 60% compared to the initial value, to a total concentration of 58.8 ± 2.0 μg/100 g fresh weight. The smallest increase, 32% of the initial value, was observed in the treatment with L. plantarum 299. Considering the average recommended intake of 250 μg dietary folate equivalents/day [24], the fermented vegetable mixture is of interest. The ability of microorganisms to produce folate is strain-specific, and a decrease in folate concentration in fermented products due to microbial consumption has been reported [25]. It should be pointed out that a significant increase in folate concentration was observed for all four strains of L. plantarum included in the present study, and that the genes for folate biosynthesis have been identified in this species [26]. Thus, fermentation of vegetables with L. plantarum might be considered as a general measure to increase folate concentration.
Vitamin B12 has a function as an important co-factor in several enzymes in procaryotes, protists, and animals, while B12-dependent enzymes have not been found in plants and fungi. Production of vitamin B12 has been shown to be limited to a few species of bacteria and archaea [27], and ensuring intake of adequate levels of this vitamin is a high concern with plant-based diets. In recent years, two strains of L. plantarum that produce vitamin B12 have been isolated [28]. In the present study, the increase in vitamin B12 in the fermentation treatments was less pronounced than that seen for riboflavin and folate. A significant increase in B12 content was observed after fermentation with L. plantarum 299 only (Table 2). An increase of 66% (to 0.048 ± 0.013 μg/100 g fresh weight) compared to the initial value (0.029 ± 0.002) was observed in this treatment. Considering that intake of 4 μg vitamin B12per day has been set as adequate by EFSA [29], it is clear that the fermented products evaluated in the present study could only provide a very small fraction of the total requirement, despite the significant increase. For the two vitamin B12-producing strains of L. plantarum previously isolated, it has been demonstrated that increased production of vitamin B12 can be achieved by addition of a B12 precursor such as 5-aminolevulinate [28]. Thus, a future approach to increase the concentration of vitamin B12 in fermented vegetables could be to ensure high concentrations of precursors before fermentation. Also, as the presence of human inactive analogues, such as pseudovitamin B12, have been reported in LAB high-producing strains should be subjected to detailed chemical analysis including not only microbiological assay but also liquid chromatographic methods [30].
It should be pointed out that no cell lysing treatment was performed in the present study, apart from storage in the freezer (−80 °C), and that strains of L. plantarum have been demonstrated to have high stability when frozen [31]. Additionally, no difference in moisture content in any of the treatments compared to the control was observed after lyophilization (data not shown). Thus, the increased levels of vitamins observed in the present study did not represent an intracellular pool, and were not due to an increase in dry matter.
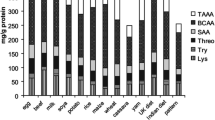
No distinguishable difference in taste could be detected in the mixture fermented with the different bacterial strains and, based on the significant increase in vitamin B12 level, the vegetable mixture fermented with strain L. plantarum 299 was chosen for analysis of amino acid composition. The results showed small increases in alanine, glycine, histidine, isoleucine, leucine, and valine in the fermented sample compared to the control (Table 3). Of these amino acids, histidine, isoleucine, leucine, and valine are essential in the human diet. Thus, fermentation with L. plantarum 299 can be considered to have slightly improved the protein quality of the vegetable mixture. In contrast, a recent study reported a decrease in protein quality after fermentation of pea proteins with L. plantarum [32]. In that study, high consumption of the sulfur-containing amino acids was observed and thus the authors recommend selection of species other than L. plantarum for fermentation. This discrepancy in results, despite working with the same species and a similar vegetable, reflects the strain-specific metabolism of L. plantarum, which has been suggested to be due to their diverse ecological niches [28]. It also highlights the need for working with several strains of the same species in order to draw sound conclusions on characteristics of the species.
Conclusions
Lactic acid fermentation is of importance for food preservation, while also having impact on taste and nutritional composition. In the present study three different vegetable substrates (cauliflower, white bean, and cauliflower-white bean mixture) were fermented using four different strains of L. plantarum. All strains had a similar impact on pH of the different substrates, and fermentation was more efficient in the treatments including cauliflower. Due to the efficient fermentation, with a final pH below 4, the pleasant taste and inclusion of legumes the impact of fermentation on riboflavin, folate, and vitamin B12 concentrations and on protein quality was studied in the cauliflower-white bean mixture. All strains of L. plantarum significantly increased the content of both folate and riboflavin compared to an unfermented control. Fermentation also had an impact on the content of vitamin B12, with fermentation with one of the bacterial strains (L. plantarum 299) resulting in a significant increase in vitamin B12 content. In the treatment involving fermentation of a cauliflower-white bean mixture with L. plantarum 299, amino acid composition was analyzed. The results revealed small increases in the concentrations of alanine, glycine, histidine, isoleucine, leucine, and valine in the fermented sample compared to the unfermented control.
Thus a slight improvement in nutritional quality was obtained after fermentation, although it should be pointed out that the quantity of different vitamins produced during fermentation, particularly of riboflavin and vitamin B12, was low relative to the recommended intake.
References
Poore J, Nemecek T (2018) Reducing food’s environmental impacts through producers and consumers. Science 360:987–992
Karlsson J, Röös E, Sjunestrand, Pira K, Larsson M, Hessellund A et al. (2017) Future nordic diets, exploring ways for sustainably feeding the Nordics. Nordic council of ministers, Copenhagen. https://doi.org/10.6027/TN2017-566
Montet D, Ray RC, Zakhia-Rozis N (2014) Lactic acid fermentation of vegetables and fruits. In: Ray RC, Didier M (eds) Microorganisms and fermentation of traditional foods. CRC Press, Florida, pp 108–140
Di Cagno R, Coda R, DeAngelis M, Gobbetti M (2013) Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol 33:1–10
Khan SA, Liu L, Lai T, Zhang R, Wei Z, Xiao J, Deng Y, Zhang M (2018) Phenolic profile, free amino acids composition and antioxidant potential of dried longan fermented by lactic acid bacteria. J Food Sci Technol 55:4782–4791
Waters DM, Mauch A, Coffey A, Arendt EK, Zannini E (2015) Lactic acid bacteria as cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: a review. Crit Rev Food Sci Nutr 55:503–520
Ducrotté P, Sawant P, Jayanthi V (2012) Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 18:4012–4018
Jansson P-A, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T et al (2019) Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol 1:e154–e162
Hoppe M, Önning G, Hulthén L (2017) Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females-double isotope sequential single-blind studies in menstruating women. PLoS One 12(12):e0189141
Molin G (2003) The role of Lactobacillus plantarum in foods and in human health. In: Farnworth RE (ed) Handbook of fermented functional foods. CRC Press, Taylor & Frances Group, Boca Raton, pp 305–342
Xu J, Ahrén IL, Prykhodko O, Olsson C, Ahrné S, Molin G (2013) Intake of blueberry fermented by Lactobacillus plantarum affects the gut microbiota of L-NAME treated rats. Evid Based Complement Alternat Med 2013:809128
Nguyen BT, Bujna E, Fekete N, Tran ATM, Rezessy-Szabo JM, Prasad R, Nguyen QD (2019) Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front Nutr 6:54. https://doi.org/10.3389/fnut.2019.00054
Jakobsen J (2008) Optimisation of the determination of thiamin, 2-(1-hydroxyethyl)thiamin, and riboflavin in food samples by use of HPLC. Food Chem 106(3):1209–1217. https://doi.org/10.1016/j.foodchem.2007.06.008
DeVries JW, Rader J, Keagy PM, Hudson CA, Angyal G, Arcot J, Castelli M, Doreanu N, Hudson C, Lawrence P, Martin J, Peace R, Rosner L, Strandler HS, Szpylka J, van den Berg H, Wo C, Wurz C (2005) Microbiological assay-trienzyme procedure for total folates in cereals and cereal foods: collaborative study. J AOAC Int 88(1):5–15
Ball GFM (2006) Vitamins in foods: analysis, bioavailability, and stability. CRC Press, Taylor & Frances Group, Boca Raton, pp 331–332
Bellomonte GA, Constantine S, Giammariolo N (1987) Comparison of modified automatic dumas method and the traditional Kjeldahl method for nitrogen determination in infant food. J Assoc Off Anal Chem 70:227–229
Llames CR, Fontaine J (1994) Determination of amino acids in feeds – collaborative study. J AOAC Int 77:1362–1402
Baloch AB, Xia X, Sheikh SA (2015) Proximate and mineral composition of dried cauliflower (Brassica oleracea L.) grown in Sindh, Pakistan. J Food Nutr Res 3:213–219
Hopper SD, Glahn RP, Cichy KA (2019) Single varietal dry bean (Phaseolus vulgaris L.) pastas: nutritional profile and consumer acceptability. Plant Foods Hum Nutr 74:342–349
Nkhata SG, Ayua E, Kamau EH, Shingiro JB (2018) Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr 6:2446–2458
Marko A, Rakicka M, Mikusova L, Valik L, Sturdik E (2014) Lactic acid fermentation of cereal substrates in nutritional perspective. Int J Res Chem Environ 4:80–92
EFSA (2017) Dietary reference values for riboflavin. EFSA J 15(8):4919. https://doi.org/10.2903/j.efsa.2017.4919
Capozzi V, Menga V, Digesu AM, De Vita P, van Sinderen D, Cattivelli L et al (2011) Biotechnological production of vitamin B2-enriched bread and pasta. J Agric Food Chem 59(14):8013–8020
EFSA (2014) Scientific opinion on dietary reference values for folate. EFSA J 12(11):3893. https://doi.org/10.2903/j.efsa.2014.3893
LeBlanc JG, Laino JE, del Valle JM, Vannini V, van Sinderen D, Taranto MP et al (2011) B-group vitamin production by lactic acid bacteria – current knowledge and potential applications. J Appl Microbiol 111:1297–1309
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA 100(4):1990–1995. https://doi.org/10.1073/pnas.0337704100
Martens JH, Barg H, Warren MJ, Jahn D (2002) Microbial production of vitamin B12. Appl Microbiol Biotechnol 58:275–285
Bhushan B, Tomar SK, Chauhan A (2017) Techno-functional differentiation of two vitamin B12 producing Lactobacillus plantarum strains: an elucidation for diverse future use. Appl Microbiol Biotechnol 101:697–709
EFSA (2015) Scientific opinion on dietary reference values for cobalamin (vitamin B12). ESFA J 13(7):4150. https://doi.org/10.2903/j.efsa.2015.4150
Chamlagain B, Sugito TA, Deptula P, Edelmann M, Kariluoto S, Varmanen P, Piironen V (2018) In situ production of active vitamin B12 in cereal matrices using Propionibacterium freudenreichii. Food Sci Nutr 6:67–76
Kandil S, El Soda M (2015) Influence of freezing and freeze drying on intracellular enzymatic activity and autolytic properties of some lactic acid bacterial strains. Adv Microbiol 5:371–382
Cabuk B, Nosworthy MG, Stone AK, Korber DR, Tanaka T, House JD, Nickerson MT (2018) Effect of fermentation on the protein digestibility and levels of non-nutritive compounds of pea protein concentrate. Food Technol Biotechnol 56:257–264
Acknowledgements
Part of this study was funded by SLU Future Food, a platform within the Swedish University of Agricultural Science developing knowledge, solutions, and innovations aimed at ensuring that the entire food system is characterized by economic, ecological, and social sustainability. We thank SLU Future Food, SLU Holdning, Partnership Alnarp and also the Swedish Food Agency for taking part in the study.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
G Önning and K Holmgren work for Probi AB, which owns the bacterial strains investigated in the study. The other authors declare that they have no conflict of interest.
Ethical Approval
This work did not involve any studies with human participants or animals performed by any of the authors.
Informed Consent
All authors have the authority to publish this material and have agreed to submit it to Plant Foods for Human Nutrition.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thompson, H.O., Önning, G., Holmgren, K. et al. Fermentation of Cauliflower and White Beans with Lactobacillus plantarum – Impact on Levels of Riboflavin, Folate, Vitamin B12, and Amino Acid Composition. Plant Foods Hum Nutr 75, 236–242 (2020). https://doi.org/10.1007/s11130-020-00806-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00806-2