Abstract
Culture-independent molecular-based approaches can be used to identify genes of interest from environmental sources that have desirable properties such as thermo activity. For this study, a putative thermo stable endoglucanase gene was identified from a mixed culture resulting from the inoculation of Brock-CMcellulose (1%) broth with mudspring water from Mt. Makiling, Laguna, Philippines that had been incubated at 90 °C. Genomic DNA was extracted from the cellulose-enriched mixed culture and endo1949 forward and reverse primers were used to amplify the endoglucanase gene, which was cloned into pCR-script plasmid vector. Blastn alignment of the sequenced insert revealed 99.69% similarity to the glycosyl hydrolase, sso1354 (CelA1; Q97YG7) from Saccharolobus solfataricus. The endoglucanase gene (GenBank accession number MK984682) was determined to be 1,021 nucleotide bases in length, corresponding to 333 amino acids with a molecular mass of ~ 37 kDa. The endoglucanase gene was inserted into a pET21 vector and transformed in E. coli BL21 for expression. Partially purified recombinant Mt. Makiling endoglucanase (MM-Engl) showed a specific activity of 187.61 U/mg and demonstrated heat stability up to 80 °C. The thermo-acid stable endoglucanase can be used in a supplementary hydrolysis step to further hydrolyze the lignocellulosic materials that were previously treated under high temperature-dilute acid conditions, thereby enhancing the release of more glucose sugars for bioethanol production.
Similar content being viewed by others
Introduction
Microorganisms and enzymes which are stable and active under extreme environmental conditions are both scientifically interesting and industrially significant for useful applications. Volcanic vents and mudspring systems located on the mountainside of Mt. Makiling, Los Baños, Laguna are potential sources of thermophilic and hyperthermophilic microorganisms. These microorganisms are excellent sources of genomic DNA for identification of genes encoding proteins with novel properties, having probable or established industrial importance.
Culture independent PCR (ciPCR) is a molecular based discovery technique which can be used to detect the presence of specific genes from non-cultured organisms in a composite multi-organic sample (DeLong and Pace 2001). This technique has previously proven its worth in ribosomal-based microbial diversity studies by (Hugenholtz and Pace 1996; Pace 1997; Handelsman et al. 2002) and in isolating bacterial genes for extracellular peptidases in soil by (Bach et al. 2001). Reference (Handelsman and Smalla 2003) believed that as little as 1% of all microbial organisms can be cultured, the independence from cultivation may enable scientists to access the total gene pool of an ecological niche. Screening for specific genes from the combined gene pool of organisms that degrade an industrially interesting substrate in nature could lead to the discovery of new enzymes with higher specific activity and substrate affinity.
Utilization of biomass for fuel alcohol production is a promising alternative to the world’s limited fossil oil reserves which are expected to be depleted due to increased energy demand. Use of this renewable energy source in fuel blends for transportation purposes could result in reduced air pollution and decreased atmospheric carbon dioxide build-up (Jacobsen et al. 2005). Cellulase, the enzyme that catalyzes cellulose degradation to glucose, is actually a complex mixture of several enzymes including endoglucanase, cellobiohydrolases (CBH, also known as exoglucanases), and β-glucosidase (Sun and Cheng 2002; Wang et al. 2009; Sadhu et al. 2013). The concerted action of these enzymes ensures an efficient hydrolysis of cellulosic materials with minimum by-product inhibition.
Enzymatic hydrolysis of cellulose to glucose is predominantly carried out by fungi, e.g. Trichoderma, Penicillium and Aspergillus at mesophilic (typically between 20 and 45 °C) temperature and slightly acid to neutral (4–7) pH conditions (Karnchanatat et al. 2008; Liu et al. 2006). To compete with the conversion of lignocellulose to glucose obtained through an acid hydrolysis method, more efficient degradation at higher temperature is needed. Dilute-acid conditions in combination with pre-treatment temperatures ranging from 180 to 200 °C can improve enzyme accessibility to the recalcitrant cellulose in pre-treated lignocellulosic feedstocks and solubilize a significant portion of the hemicellulosic component.
The use of thermostable and thermoactive enzymes in lignocellulose hydrolysis is promising because it can achieve high sugar yields and eliminate the need for large quantities of chemicals and the formation of inhibitory by-products during dilute acid hydrolysis (Tran and Chambers 1986). This study was undertaken to enable the cloning of a thermostable endoglucanase gene directly from the genomic DNA of the cellulose-enriched mixed culture originally obtained from the Mt. Makiling mudspring and to subsequently express the cloned endoglucanase gene in Escherichia coli to enable its biochemical characterization.
Materials and methods
Mixed culture
One liter of Brock broth medium (Brock et al. 1972) containing 1% carboxymethylcellulose (BBM-CMcellulose) as the carbon source was inoculated with combined mudspring water and mud from Mt. Makiling, Los Baños, Laguna, Philippines at a 10% inoculum level. Sterilized mineral oil was added to the inoculated medium and incubated at 90 °C for at least 1 month. The effect of pH on the formation of pellicle at the oil–water interface was studied by preparing a big volume of the BBM-CMcellulose and adjusting the pH of the specific volume to the desired pH using 1 M HCl before sterilization.
Bacterial strain, culture conditions and plasmids
Escherichia coli strain EC XL-1 Blue (Stratagene, La Jolla, CA, USA) and pPCR-Script plasmid (Novagen, Madison, WI, USA) were used for plasmid construction and sequencing. Escherichia coli strain BL21(DE3) (Stratagene, La Jolla, CA, USA) and pET21a+ plasmid (Novagen, Madison, WI, USA) were used for plasmid construction and expression. Transformant E. coli strains were selected on LB plates containing 1.5% agar (w/v) and 50 µg/ml penicillin. E. coli cultures were grown in LB broth (Sambrook et al. 1989) at 37 °C in a shaking (2.50×g) incubator.
Preparation of the mixed culture for DNA extraction
The mineral oil layer above the pellicle layer was aseptically removed using a sterile pipette tip. The pellicle layer was gently collected in an Eppendorf tube (1.5 ml) using a pipette tip with enlarged hole for centrifugation at 2500×g. After centrifugation, most of the remaining oil on the surface of the pelleted biomass aggregate was gently sucked out with a pipette tip. The precipitate was used for genomic DNA extraction.
DNA extraction
The genomic DNA of the pellicle from the cellulose enriched mixed culture that collected at the mineral oil-liquid interface was extracted using the cetyltrimethylammonium bromide (CTAB)/sodium chloride (NaCl) method (Maniatis et al. 1982), aluminum sulfate method (Dong et al. 2006), and the commercially available MoBio PowerSoil DNA Isolation kit with some modifications (MoBIO, QIAGEN INC., Maryland, USA). Samples of DNA preparations were diluted accordingly and read in a Nanodrop spectrophotometer set at A260 nm using the Smart Spectrophotometer 3000 (BioRad, Hercules, CA). The concentration (ng/µl) and quality of the resulting genomic DNA obtained from different extraction procedures were compared based on its A260/A280 ratio and A260/A230 ratio.
PCR procedure
The endoglucanase gene was amplified from the extracted genomic DNA by Polymerase Chain Reaction (PCR) using the following primers: sso1949F: 5′-ATGAATAAGCTTTATATTGTGCTTCCGGTAA-3′ (HindIII site in bold) and sso1949R: 5′-AAGGTCCTCGAGTCTTATATTGTTTAGAGG-3′ (XhoI site in bold) (Brouns et al. 2005) and EngluF: 5′-GCTAGCGCTATTTACCTACACC-3′ (NheI site in bold) and EngluR: 5′-AAGCTTAGAGGAGAGTTTCAGAAAAGTTGG-3′ (HindIII site in bold) (Huang et al. 2005). The primers created restriction cleavage sites upstream and downstream of the target gene. Each PCR reaction was performed in a total volume of 40 μl which included 0.5 μl of IProof High Fidelity DNA polymerase, 0.5 μl of 0.2 nM dNTPs, 0.2 μl each of 0.2 μM endo1949 primers, 10 μl of 5X HF reaction buffer containing 1.4 mM MgCl2 and the volume was adjusted with molecular biology grade water. PCR amplification was done with a Thermal Icycler (BIORAD, California, USA) using the following PCR program: initial denaturation at 98 °C for 30 s, 30 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s. Final extension was at 72 °C for 7 min. After the PCR run, the PCR mixtures were loaded on to prepared agarose gels (1.0%) augmented with ethidium bromide (5 μg/μl), run through the electrophoresis set-up at 80 V and visualized on a UV transilluminator.
Cloning procedure
The purified PCR product of ~ 1.0 kb length obtained using the sso1949F/R primers for Brouns et al. (2005) was ligated in to the pPCR-Script vector (3000 bp) (Novagen, Madison, Wisconsin, USA) by blunt end ligation using the EcoRV restriction site. The EcoRV restriction site in the Multiple Cloning Site (MCS) region of pPCR-Script plasmid was utilized as the insertion region of the PCR amplicon. Blunt-ended cloning procedures capture blunted PCR fragments for bidirectional insertion. As with the terminal deoxynucleotide transferase activity (T/A) system, blunt-ended cloning does not require the addition of extra bases to the primer sets, thereby allowing pre-existing primers to be used to generate and clone a DNA fragment (Costa et al. 1994). The resulting plasmid was transformed into competent E. coli XL-1 blue cells using the calcium chloride transformation method (Maniatis et al. 1982). The clones were confirmed through PCR and restriction analysis. The purified recombinant plasmid coded as pPCR-Engl-24 was digested with the restriction enzymes HindIII and XhoI (New England, BIOLABS, Massachusetts, USA) and purified restricted DNA fragment was ligated into a HindIII/XhoI restriction digested plasmid pET21a(+) (5443 bp) (Novagen, Madison, Wisconsin, USA) and resulting recombinant plamids were designated as pEngl-24-21. The ligated pEngl-24-21 plasmid was transformed into competent cells of E. coli BL21(. The clones were confirmed through PCR and restriction enzyme digestion analyses.
Nucleotide sequencing
QIAGEN purified recombinant pEngl-24 plasmids were used as templates for nucleotide sequencing using the universal T3 and T7 primers. The plasmids (150–300 ng/μl) were submitted to Eurofins MWG Operon (Huntsville, Alabama, USA) for nucleotide sequencing. Sequence data were aligned, analyzed and compared using the Blastn software package for determination of possible identity of the inserted DNA fragment (https://www.ncbi.nlm.nih.gov/BLAST). The nucleotide sequence was translated using the NCBI database (https://web.expasy.org/cgi-bin/translate/dna_aa).
The amino acid sequence of the translated pEngl-24 was compared against other glycosyl hydrolase family 12 endoglucanases found in the NCBI database by Multiple Sequence alignment. The deduced pEngl-24 sequence was aligned against endoglucanases, sso1354 (CelA1; Q97YG7), sso1949 (CelA2; Q97XO8) and sso2534 (CelB; Q97VS7) from Saccharolobus solfataricus, SiH_0339 (ADX81754.1) from Sulfolobus islandicus, Igag_0454 (ADM27292.1) from Ignisphaera aggregans, Cel12A (Q9S5X8) from Thermotoga maritima and CelA (O33897) from Rhodothermus marinus. The FASTA format of the amino acid sequences were downloaded from the UniProt website (https://www.uniprot.org/).
The correct reading frame of the amino acid sequence was verified using TBBLASTX analysis (https://www.ncbi.nlm.nih.gov/BLAST). QIAGEN purified recombinant pEngl-24-21-63 plasmids were also submitted to Eurofins MWG Operon for confirmation of the nucleotide sequence using the universal T7 terminator primer.
Recombinant expression of the thermostable endoglucanase in E. coli BL21(DE3)
The seed culture of the transformant E. coli BL21 containing pEngl-24-21 plasmid was made by inoculating a 5 ml Luria Bertani broth medium containing ampicillin (100 μg/ml). The culture was incubated at 37 °C for 18–20 h with shaking (2.50×g). The auto-induction medium (200 ml) of Studier (2005) was prepared in a 500 ml Erlenmeyer flask and sterilized at 121 °C for 15 min. The seed culture (~ 5 ml) was inoculated into 200 ml of medium aseptically. Likewise, ampicillin was added to the medium right before inoculation of the production medium. The inoculated flask was incubated at 37 °C with shaking (2.50×g) for 16 h.
After the prescribed incubation time, 100 ml of the culture broth was aseptically inoculated to the sterilized 2.0 l medium in a 4.0 l Erlenmeyer flask. The inoculated flask was incubated at 37 °C with shaking (2.50×g). The culture broth was harvested after the prescribed incubation time and transferred to sterile centrifuge tubes for centrifugation at 10,000×g for 15 min at 4 °C. The supernatant was collected in a sterile container while the biomass pellets were placed inside an autoclavable plastic bag for decontamination.
Preparation of protein samples
The protocol of the Grunden laboratory for the preparation of recombinant proteins for small scale expression studies was followed (Grunden 2011). From the autoinduction expression media (Studier 2005), separate 1.0 ml volumes of the culture broth were placed in Eppendorf tubes (1.5 ml) for a total of 3 tubes per medium. The tubes were centrifuged at 10,000×g for 10 min at 5 °C. The supernatant from all the tubes were removed by pipetting out and discarded.
To prepare whole cell extract (WCE), the bacterial pellet was resuspended in 50 μl of 50 mM potassium phosphate (pH 7.0), 0.05 μl 1 M dithiothreitol (DTT) and 0.05 μl 1 M benzamidine (Grunden 2011). The aforementioned components were added to lyse the cells, disrupt the cell wall and membrane and release all the proteins made in the cell bringing all of them in suspension whether cytoplasmic, periplasmic or insoluble.
Cell free extract (CFE) was prepared by resuspending the bacterial pellet in 300 μl B-PER reagent (Invitrogen, USA) containing ~ 0.1 g of glass disruption beads (0.1 mm diameter). The tube was placed on a MoBio shaker platform for 15 min to disrupt the cell wall and membrane and release the proteins in the cytoplasm. The tube was centrifuged at 10,000×g for 10 min, 5 °C and the resulting supernatant was carefully transferred to a new centrifuge tube.
To prepare the heat stable (HT) suspension, 100 μl of the harvested supernatant CFE was placed in an Eppendorf tube and incubated for 15 min at 70 °C. After the incubation time, the tube was centrifuged at 10,000×g for 10 min, 5 °C and the resulting supernatant was carefully transferred to a new centrifuge tube. This consisted the heat stable, soluble proteins from the cytoplasm.
For the periplasm portion, the bacterial pellet was resuspended in 1.5 ml of 30 mM Tris–HCl, 20% sucrose (pH 8.0) solution (Grunden 2011). After thorough resuspension, 0.5 μl of 0.5 M ethylenediaminetetraacetic acid (EDTA) (pH 8.0) was added and the tube was shaken for 10 min (2.50×g) at ambient room temperature (2.50×g). The tube was centrifuged at 10,000×g for 10 min at 5 °C after which the supernatant was discarded. The pellet was resuspended in 1.5 ml ice-cold 5 mM MgSO4 and shaken (2.50×g) at 4 °C for 10 min. After the incubation time, the tube was centrifuged at 10,000×g for 10 min, 5 °C and the resulting supernatant was carefully transferred to a new centrifuge tube. The tube contains proteins that were being transported across the cell membrane for secretion and those proteins that are not fully secreted to the medium.
The remaining culture broth of the expression medium was transferred to a conical tube and centrifuged at 9500×g for 20 min, 4 °C. The supernatant was collected and coded as “Culture supernatant” that contained all the proteins transported outside the cell during growth. The bacterial pellet that precipitated was discarded.
Ammonium sulfate precipitation
Ammonium sulfate crystals were added to the culture supernatant to an 80% saturation to precipitate the target endoglucanase protein then incubated at 4 °C overnight (Yin et al. 2010; Dave et al. 2015). The solution was centrifuged at 10,000×g for 20 min, 4 °C to separate the supernatant containing the ammonium water complex from the precipitated proteins.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The samples were subjected to SDS-PAGE using a Mini-Protein II system (BIORAD, California, USA). Ten (10) μl each of the protein samples (WCE, CFE, HT, Periplasm and Culture supernatant) were added to 10 μl of 2X Protein Dye (0.05% bromophenol blue, 5% β-mercaptoethanol, 10% glycerol, and 2% SDS in 0.25 M Tris–HCl buffer; pH 6.8) and boiled at 100 °C for 20 min. After the boiling period, all the samples in the tubes were loaded into separate wells of the protein gels. Electrophoresis was performed at room temperature for ~ 45 min at 200 V according to the method described by Laemmli (1970).
Substrate specificity
The hydrolytic ability against 1% CMcellulose, cotton, filter paper and xylan in 20 mM Na2HPO4/citric acid buffer (pH 3.0) incubated at 80 °C for 30 min was determined to evaluate the substrate specificity of the partially purified endoglucanase. The activity was measured according to the procedure of Miller (1959).
Enzyme assay and measurement of protein content
Endoglucanase activity was determined by measuring the amount of reducing sugar released from CMcellulose (SIGMA, Missouri, USA) after enzyme treatment using dinitrosalicylic (DNS) acid reagent (Miller 1959). The standard assay mixture contained 100 ul of the enzyme and 900 μl of 1% CMcellulose in 20 mM Na2HPO4/citric buffer (pH 3.0). The mixture was incubated at 50 °C. After 30 min reaction, 1 ml of DNS was added and boiled in a water bath for 5 min to stop the reaction. The resulting samples were cooled to room temperature and absorbance was measured at 540 nm (A540). One unit of endo-β-1,4-glucanase activity is defined as the amount of enzyme that could hydrolyze CMcellulose and release 1 μg of glucose within 1 min reaction at 50 °C (Miller 1959). Enzyme assays were carried out in duplicate for each treatment.
Protein concentration was determined by the method of Lowry et al. (1951) using bovine serum albumin (BSA) as standard.
Results
Microbial culture
In the initial set-ups, the addition of mineral oil (up to ~ 0.5 cm thickness) to Brock-CMC medium in serum bottles covered with sterilized rubber caps after inoculation, minimized the evaporation of the broth during prolonged incubation at 90 °C. A mixed culture was able to grow at 90 °C. The growth of the microorganisms within the broth was slow, and increased turbidity of the broth due to suspended microbial growth and pellicle formation at the mineral oil-liquid interface occurred after 2 weeks of incubation at 90 °C, while clumps at the bottom part of the vessel were observed at a later time (Fig. 1).
Growth of the mixed culture in Brock’s et al. (1972) medium (pH 3.5) containing 1.0% carboxymethylcellulose (CMcellulose) as substrate. The culture vessels were sealed with a rubber cap and crimped with an aluminum seal to minimize evaporation of the medium while the cultures were incubated at 90 °C for 2 months. a picture of the cultures showing the aluminum seal and needle, b close up view of the inoculated broth after 2 months incubation showing the (1) mineral oil layer, (2) pellicle formed, (3) clearing of the broth, and (4) clumps at the bottom of the vessel
The initial set-up was modified due to the positive pressure generated by the heat of the medium which caused the rubber cap to sometimes loosen or be removed from the bottle. Likewise, direct exposure of the culture broth to the high temperature (90 °C) caused the evaporation of the liquid even if a mineral oil layer was present, leading to the decrease in volume of the culture broth. By crimping with aluminum seal and insertion of a sterile 1 inch 25-gauge needle through the rubber cap, the pressure that built-up was prevented from accumulating inside the bottle (Fig. 1). Moisture that accumulated at the upper portion of the bottle eventually dropped back into the medium thus minimizing the decrease in volume.
Effect of pH
Clump formation and clearing of the broth were most evident in the medium adjusted to pH 1.0 and 2.0 before sterilization while formation of pellicle at the oil-medium interface was more visible in the pH 3.0 and 4.0 cultures (Fig. 2).
Growth of the mixed culture at different pH of the Brock (1972) medium containing 1.0% carboxymethylcellulose (CMC) as substrate incubated at 90 °C for 1 month. a uninoculated medium, b inoculated medium, c close-up view of the inoculated medium showing the coagulated biomass at the different pH values
Amplification of the endoglucanase gene using gene-specific primers
The concentration and quality of genomic DNA obtained by the CTAB method was comparable to the genomic DNA obtained using the commercial MoBio Powersoil kit while a minimal amount of genomic DNA was obtained using the aluminum sulfate method described by Dong et al. (2006). Thus, the CTAB method was employed for the extraction of the genomic DNA of the cellulose enriched mixed culture used for amplification with gene-specific primers.
Temperature gradient PCR amplification using the genomic DNA from the mixed culture as template and primer pairs, 1) sso1949F/R based on Brouns et al. (2005) and 2) englu1F/R based on Huang et al. (2005) gave ~ 1.0 kb and ~ 0.8 kb PCR products, respectively (Fig. 3). The group of Huang et al. (2005) used the above mentioned sso1949F/R primer pair to amplify the region of the SSO1949 gene encoding the hypothetical extracellular cellulase, while the sso1949F/R primer pair was used by the group of Brouns et al. (2005) to construct the ORF sso1949 from Sulfolobus solfataricus P2 genomic DNA by PCR amplification. Better amplification was obtained from the sso1949F/R primers of Brouns et al. (2005) as compared to the englu1F/R primer pair of Huang et al. (2005) based on the intensity of the ~ 1.0 kb amplification band. For sso1949F/R, clean PCR product and high concentration of the target amplification band was obtained at the annealing temperature range of 59.8 to 55.0 °C. The annealing temperature of 59.8 °C was used for succeeding PCR amplifications to prevent the occurrence of non-specific amplification.
Insertion into pPCRscript vector
The ~ 1.0 kb length amplicons that corresponded to the endoglucanase gene that had been cloned into the pPCRscript plasmid and transformed into E. coli XL-1 Blue resulted in several transformant colonies. Recombinant plasmids positive for insertion of the ~ 1.0 kb PCR product using colony PCR and sso1949F/R primers were coded as pEngl. The presence of the insert was confirmed by comparing the molecular sizes of the (a) recombinant pEngl plasmids and the pPCRscript plasmid, (b) single restriction digestion with HindIII to evaluate the molecular mass of the linearized recombinant pEngl plasmid and pPCRscript, and (c) PCR using the recombinant pEngl plasmid and pPCRscript as template and the sso1949 primers to amplify the ~ 1.0 kb product. The presence of the insert was confirmed by comparing the molecular sizes of the recombinant pEngl plasmids (~ 2.8 kb) and the pPCRscript plasmid (~ 1.8 kb), wherein the recombinant plasmids were bigger by ~ 1.0 kb than the pPCRscript plasmid which corresponds to the ~ 1.0 kb size of the inserted fragment. Single restriction digestion of the recombinant pEngl plasmids with HindIII gave a molecular size of ~ 4.0 kb which is the approximate total of the restricted ~ 3.0 kb pPCRscript plasmid and the ~ 1.0 kb inserted amplicon. Lastly, after PCR using the recombinant pEngl plasmid and the pPCRscript plasmid vector as template and the sso1949 primers, the ~ 1.0 kb amplicon was obtained from the reaction mixtures of the recombinant plasmids alone.
Nucleotide sequencing and identity analysis
The nucleotide sequence of the gene inserted in pEngl-24 was deduced from the alignment to have 1,021 nucleotide bases which corresponds to the ~ 1.0 kb molecular size of the PCR fragment that was inserted into the plasmid. Nucleotide sequence query of the pEngl-24 gene resulted to 99.69%, 99.5% and 93.90% identity with Sulfolobus solfataricus P2 complete genome (AE 00664.1), Sulfolobus solfataricus P1 genome (LT 549890.1) and Sulfolobus shibatae B12 ssglu gene for endo-1,4-β-glucanase (LT 221867.1) from the NCBI database, respectively (data not shown).
Using ExPASy program, the nucleotide sequence was translated into the protein complement through its amino acid sequence (https://web.expasy.org/cgi-bin/translate/dna_aa) (Fig. 4). The entire endoglucanase insert is composed of 335 amino acid residues including the STOP codon of the open reading frame (ORF). Initial BLAST query of the translated amino acid sequence showed that the sequence had a very high % identity with Saccharolobus solfataricus endoglucanase, formerly named Sulfolobus solfataricus (Sakai and Kurosawa 2018).
The nucleotide sequence of the endoglucanase gene contained in the pEngl-24 plasmid that was deduced from the alignment of sequences obtained from T7 forward and T3 reverse primers together with the deduced amino acid sequence of the recombinant endoglucanase as translated from the nucleotide sequence by ExPasy program (https://www.web.expasy.org/cgi-bin/translate/dna_aa). The deduced amino acid sequence is represented by one-letter amino acid symbols shown below the DNA sequence. Included in the figure are the positions of the forward and reverse primers used to amplify the endoglucanase gene. The position of the putative signal peptide is indicated by a box, light green shade denotes the Serine-Threonine rich linker region while the green shade denotes the ORF of the endoglucanase gene. The catalytic glutamate residues (G213 and G310) are encircled
The works of Brouns et al. (2005), Huang et al. (2005) and Girfoglio et al. (2012), showed that Saccharolobus solfataricus has three endoglucanase genes, sso1354, sso1949 and sso2354, all belonging to glycosyl hydrolase (GH) family 12. The translated sequence of pEngl-24 also showed part of the signal peptide and the entire Ser-Thr rich linker region described by Huang et al. (2005) in the sso1949 sequence (Fig. 5). The catalytic glutamate residues typical for glycosyl hydrolase family 12 and described by Huang et al. (2005) in sso1949 and Girfoglio et al. (2012) in sso1354 were also present as Glu214 and Glu310 in our sequence. The nucleotide sequence for pEngl-24 has been submitted to the NCBI database and had been provided with the GenBank accession number MK984682.
Homology between the deduced amino acid sequence of the endoglucanase contained in pEngl-24 plasmid against endoglucanases from Saccharolobus solfataricus (sso 1354, sso1949 and sso2534), endoglucanase from Sulfolobus islandicus, Ignisphaera aggregans, Thermotoga maritima and Rhodothermus marinus. Box A indicate the putative signal peptide, box B indicate the Serine-Threonine rich linker region. Asterisks indicate the catalytic glutamate residues, while the arrow indicates the potential amino acid residue responsible for the low pH optima of the protein. The position number of each amino acid sequence is indicated on both sides while the identity of the sequences is on the left
Alignment of the deduced amino acid sequence of the translated pEngl-24 gave 100% identity with sso1354, 84.79% for SiH_0339 and 83.82% with sso1949. The sequence gave 48.48%, 33.2%, 25.91% and 34.52% identities with Igag_0454, Cel12A, CelB and CelA, respectively. The signal peptide segment and Ser-Thr rich linker region were conserved for the nucleotide sequence of the endoglucanase cloned in this study hereby designated as Mt. Makiling endoglucanase protein or MM-Engl, sso1354, S. islandicus, sso1949 and Ignisphaera aggregans. gen. nov., sp. nov. (=DSM 17230T=JCM 13409T). I. aggregans represents the type strain of a novel species of a new genus within the Crenarchaeota. Just like Saccharolobus, it is a hyperthermophile, with an optimal temperature for growth between 92 and 95 °C, but unlike Saccharolobus which grows optimally at pH 2–4, it is a moderate acidophile, with optimal growth occurring at pH 6.4 (range 5.4–7.0) (Huang et al. 2005; Niederberger et al. 2006). The amino acid sequence of the cloned MM-Engl protein places the two catalytic glutamate residues, namely Glu214 and Glu310 in positions that are equivalent to that of catalytic glutamate residues of the other glycosyl hydrolase family 12 sequences (Huang et al. 2005), where Glu214 acts as nucleophile while Glu310 is the acid/base catalyst.
Insertion into pET-21a(+) vector and expression
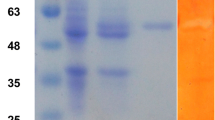
The DNA fragment containing the endoglucanase gene was successfully inserted within the multiple cloning site (MCS) region of the pET-21a(+) expression vector. Growing the transformant E. coli BL21 in auto-induction medium, allowed the endoglucanase gene contained in pEngl-21-63 to be expressed with a molecular size of ~ 37 kDa. After comparing the banding patterns of the E. coli BL21 pEngl-24-21-63 and E. coli BL21 without the recombinant plasmid, the ~ 37 kDa band was shown to be present in all the banding patterns of the E. coli BL21 pEngl-24-21-63 plasmid (Fig. 6). The said protein band did not appear in the banding patterns of the E. coli BL21 without the recombinant plasmid even after auto-induction. The expressed protein is secreted to the medium based on its presence in the supernatant. The target protein band was still present in the banding pattern of the heat treated (85 °C for 30 min) cell extracts (Fig. 7).
Comparison of protein bands from Escherichia coli BL21 cultures containing pET21a(+) plasmid vectors without and with endoglucanase insert grown in auto-induction medium. BL21, E.coli BL21 containing pET21 without insert; 16-1, E. coli BL21 containing pEngl-24-21-16; M, molecular weight marker. Lane: 1 = whole cell extract (WCE) from E. coli BL21 containing pET21 plasmid without insert; 2 = whole cell extract (WCE) from transformant E. coli BL21 16-1 containing pEngl-24-21-16; 3 = cell free extract (CFE) from transformant E. coli BL21 16-1 containing pEngl-24-21-16; 4 = CFE from transformant E. coli BL21 pEngl-24-21-16 clone heat treated (HT) at 85 °C for 30 min; 5 = periplasm from transformant E. coli BL21 pEngl-24-21-16 clone; and 6 = supernatant (S) from transformant E. coli BL21 pEngl-24-21-16 clone grown in auto-induction medium
Small scale expression studies of Escherichia coli BL21 pEng-24-21-63-4. BL21 = E. coli BL21 containing pET21 without insert; M = molecular weight marker Lane 1 = whole cell extract (WCE); 2 = cell free extract (CFE); 3 = CFE heat treated (HT) at 85 °C for 30 min; 4 = periplasm; 5 = supernatant (S) from culture broth; 6 = supernatant heat treated (HT) at 85 °C for 30 min; 7 = precipitate after ammonium sulfate precipitation of heat treated supernatant; and 8 = supernatant from ammonium sulfate precipitation step
CMCase activities using different substrates
Volumetric CMCase activities of the partially purified endoglucanase assayed using different substrates namely CMcellulose, filter paper, cotton and xylan differed among the various substrates tested (Fig. 8). Highest activity was obtained using CMC. Enzyme activities obtained for xylan and filter paper were almost similar while a very low enzyme activity was obtained when cotton was used as substrate.
Discussion
It is well known that thermophiles have very rich diversity; however, studies on them in natural environment are not well documented due to the extreme environmental conditions (e.g. high temperature and low pH) required for isolating and growing the microorganisms which limit the in vitro cultivation of the prevailing microflora and their subsequent utilization (Lantican et al. 2011). Culture-independent molecular-based techniques could potentially prove useful as an alternative enzyme discovery strategy, thus enabling scientists in exploring microbial communities and access to the total gene pool of an ecological niche like extreme environments (Handelsman 2004).
Incubation of the inoculated experimental bottles containing Brock-CMC medium at 90 °C provided the high temperature requirement of the thermophilic microorganisms in the samples from Mt. Makiling mudspring. However, prolonged incubation at the high temperature enhanced the evaporation of the medium which immediately lowered the volume. The use of mineral oil layered on top of the liquid mineral medium mitigated the evaporation rate of the set-up. The oil layer was not thick enough to enhance the onset of anaerobic conditions in the set-up.
The CMcellulose in the liquid mineral salts medium as well as the mineral oil added is a complex mixture of carbon compounds and hydrocarbons, such that a consortium or mixed culture can more effectively degrade it rather than a single isolate as the organisms complement each other through interdependence and synergistic interactions among members of the associations (Cerqueira et al. 2011; Mukred et al. 2008). In a mixed culture, enhanced microbial growth and metabolism require energy as well as the components needed for growth, with the requirements differing from organism to organism resulting to metabolic byproducts and physical alterations in the oil and liquid phases. Bacteria from the mudspring sample will grow at the water–oil interface since they need nutrients and carbon source from the components of the oil and liquid mineral salts medium. Reduction in interfacial tension (IFT) by bacterial action allow the cells to grow and divide at the interface and sustain the growth on hydrocarbons under both aerobic and anaerobic conditions, through sulfate- and nitrate respiration for the latter (Kowalewski et al. 2004).
Strong adhesion to the oil–water interface and an affinity for each other leads to an assembly of hydrophobic bacteria at the oil–water interface that resists coalescence and deformation. The observation of self-assembly of bacteria at the oil–water interface was demonstrated by the group of Dorobantu et al. (2004) for the test organisms, Acinetobacter venetianus RAG-1 and Rhodococcus erythropolis 20S-E1-c.
Since the source of the sample originated from an acid solfatara with in situ temperature ranging from 70 to 90 °C (Lantican et al. 2011), pH values ranging from 2 to 5 (Bundac et al. 1976) and a high salinity dominated by iron and sulfate (Oles and Houben 1998) with CMcellulose in the medium, acid tolerant thermophilic cellulase producers were cultivated. The organisms able to survive the low pH levels, high temperature incubation and metabolize the CMcellulose substrate, grew and multiplied leading to the formation of the bacterial pellicle at the mineral oil–water interface. The added CMcellulose might have been primarily metabolized by the organisms since minimal or no emulsification of the oil occurred as indicated by the absence of perforation or decrease in the thickness of the mineral oil layer.
The clumping at the bottom of the broth could be fermentation products, aggregates of CMcellulose and partial degradation products. From an initially dark color, the broth turned to a yellowish color with increased turbidity which could have signified the degradation of the soluble and suspended CMcellulose substrate and increase in suspended microbial cell population. Change in color of the medium, especially for media at pH 3.0, 4.0 and 5.0, was evident along with reduction of coagulation observed as the pH increased. The clumps at the bottom part of the broth were produced later on.
Even though pellicle formation and clumping were also observed at pH 5.0 of the medium, the growth at pH 3.0 and 4.0 were more significant for the objectives of this study. The ability of the culture to grow at acidic (pH 3.0–4.0) and high temperature (90 °C) using CMcellulose as substrate showed that the cellulolytic enzyme system of the organism was active. This is very important for future applications in hydrolytic processes wherein acid-high temperature stability are required for the hydrolysis of lignocellulosic materials.
Direct isolation of the genes directly from a mixed culture is a challenge for molecular microbiologists since a majority of environmental microorganisms (~ 99%) cannot be cultured in a laboratory (Amann et al. 1995). A culture independent PCR (ciPCR) molecular based discovery technique has been developed to detect the presence of genes from environmental samples, including uncultured microbes (Jacobsen et al. 2005). The independency of cultivation could potentially prove useful as an alternative enzyme discovery strategy, thus enabling scientists to access the total gene pool of an ecological niche. This technique has previously proven its worth in ribosomal-based microbial diversity studies (Hugenholtz and Pace 1996; Pace 1997; Handelsman et al. 2002) and has been used to discover a remarkable number of novel biocatalysts (Simon and Daniel 2009), including cellulases (Voget et al. 2003). This ciPCR approach was proven effective in this study with the successful isolation of the endoglucanase gene using the extracted DNA from the pellicle of the mixed culture obtained in the experimental set-up. The constructed cellulase expression cassette from the amplified endoglucanase gene using the published sense and anti-sense primers specific for sso1949 gene (Brouns et al. 2005) is similar to that reported by Huang et al. (2005) for sso1949 and Girfoglio et al. (2012) for sso1354 genes.
The works of Brouns et al. (2005) and Huang et al. (2005) for sso1949 and Girfoglio et al. (2012) for sso1354 genes used genomic DNA extracted from a pure culture of Sulfolobus solfataricus P2 as template for PCR amplification. They used sense and anti-sense primers specific for sso1949 and sso1354 genes encoding for the hypothetic extracellular cellulases SSO1949 and SSO1354 but containing sequences modified for restriction enzyme sites upstream and downstream of the genes, respectively.
The technique, ciPCR is an alternative to the otherwise well-established cultivation-based enzyme discovery strategy. In the search for new enzymes genes originating from poorly culturable and less dominant microorganisms could be recovered (Hugenholtz and Pace 1996; Dawson and Pace 2002) potentially leading to discovery of enzymes with higher substrate affinity and specific activity.
Using oligonucleotide primers designed for use in a culture-independent PCR screening, amplified clone sequences affiliated with glycosyl hydrolase family were obtained by Jacobsen et al. (2005) using DNA from composite fungal communities, inhabiting residues of corn stovers and leaves. The work of Edwards et al. (2008) cloned cellobiohydrolase, cbhI genes from basidiomycete and ascomycete fungi, forest floor and mineral soil. Cucurachi et al. (2013) directly obtained eight (8) genes encoding for cellulolytic enzymes by direct PCR amplification of genomic DNA recovered from woodland soil samples.
Cloning efficiencies of polymerase-generated PCR products can be increased by using T4 or Pfu DNA polymerase to polish or complete unfinished fragments as well as remove any added extended nucleotides. In general, T4 DNA ligase concentrations can be optimized to achieve > 70% completion of clonal insertion in 1–2 h at room temperature (Costa et al 1994). Unfortunately, blunt-ended cloning is an inefficient method, with recombinant insertion generally accounting for ~ 10% of all transformants. To alleviate this drawback, phenotypic selection [e.g., disruption of the [β-galactosidase (β-Gal) gene] is often used to detect recombinants (white colonies on X-gal containing agar plates) from non-recombinants (blue colonies on the above plating medium). Inclusion of a restriction enzyme (in this case EcoRV) in the ligation reaction increased the efficiency by maintaining a high-steady-state concentration of digested vector DNA (Costa et al. 1994; Stratagene, USA). This strategy was used and proven successful as shown by the presence of the nucleotide sequence of the gene (deduced from the alignment to have 1,021 nucleotide bases) inserted in pEngl-24 which corresponds to the ~ 1.0 kb molecular size of the PCR fragment that was inserted into the plasmid.
The expressed protein with an approximate subunit molecular mass of ~37 kDa was in good accordance with the molecular mass of ~37 kDa or 37,386 Da calculated from the deduced amino acid count of 333 (pepstart-EMBOSS program https://www.bioinformatics.nl/emboss-explorer). The amino acid count determined from the recombinant endoglucanase was close to the hypothetic endo-β-glucanases, SSO1949 encoded by 334 amino acids and SSO1354 encoded by 335 amino acids, which were cloned from the thermoacidophilic archaeon, Sulfolobus solfataricus P2 by Huang et al. (2005) and Girfoglio et al. (2012), respectively.
The molecular size of the recombinant endoglucanase was close to the molecular mass of a cellulase from Thermoascus aurantiacus RBB-1 at 35 kDa (Dave et al. 2015) and a thermostable endoglucanase isolated from the wood-decaying fungus Daldinia eschscholzii of 46.4 kDa as determined by SDS gel electrophoresis (Karnchanatat et al. 2008). This molecular mass was similar with some other low molecular mass endo-glucanases (25–45 kDa), such as 27 kDa from Mucor circinelloides (Saha 2004), 35 kDa from Chalara paradoxa (Lucas et al. 2001), 40 kDa from Bacillus strains (Mawadza et al. 2000) and 40 kDa from Aspergillus niger (Akiba et al. 1995). An extracellular endoglucanase from culture filtrates of Aspergillus awamori strain F18 was revealed to be a monomeric enzyme with a molecular weight of about 43 kDa (Singh et al. 2011).
In contrast, a thermotolerant endoglucanase with a molecular weight of 97 kDa as determined by SDS-PAGE was obtained from a Bacillus sp. isolated from cow dung (Sadhu et al. 2013). Likewise, the endo-l, 4-β-d-glucanase isolated from the genomic DNA of the saccharolytic thermophilic anaerobic bacteria, Caldicellulosiruptor bescii DSM 6725 and expressed in E. coli BL21, corresponds to a protein of 755 amino acid residues, with a calculated molecular weight of 82.154 kDa (Bista 2011). In the study of Bok et al. (1998), two thermostable endocellulases, CelA (molecular mass, 29 kDa) and CelB (molecular mass, 30 kDa) purified from Thermotoga neapolitana had lower molecular masses as compared against the recombinant endoglucanase, MM-Engl of the study.
The thermostability of the expressed ~37 kDa protein observed in the cell extract which was still structurally intact even after the heat treatment (85 °C for 30 min) appears to be of significance in its utility in the industry. These high-temperature operating cellulases with high specific activity and increased flexibility can be used in all processes in which hydrolysis of fibrous crystalline cellulosic biomass materials at high temperatures is desired. In the current industrial processes, cellulolytic enzymes are employed in detergents, causing color brightening and softening, stoning of jeans, pretreatment of cellulosic biomass to improve nutritional quality of the forage and in the pretreatment of industrial wastes (Kikani et al. 2014). For these reasons they could work at low dosages and the higher working temperatures could speed up the hydrolysis reaction time. As consequence, the overall process costs could be reduced. Thermostable enzymes could play an important role in assisting the liquefaction of concentrated biomass suspensions at high temperatures to produce high-sugar containing fermentation broths necessary to achieve ethanol concentrations in the range 4–5 wt% (Verardi et al. 2012). The partially purified endoglucanase enzyme, Mt. Makiling mudspring-endoglucanase (MM-Engl) with a volumetric activity of 13,569 U/g, and with a protein content of 72.33 mg/gram sample, had a specific activity of 187.61 IU/mg protein comparable to the 95.57 IU mg−1 (soluble protein)−1 specific enzyme activity obtained from the production technique of Duan et al. (2004) using Trichoderma pseudokoningii S-38 as cellulase producer with glucose as carbon source and cellulose fiber (CF)11 as inducer for the production of EG for 88 h fermentation time.
Among the substrates tested, highest activity was obtained using CMC which is a water-soluble long-chained cellulose with carboxymethyl substitutions. It is commonly used as a model substrate for detecting β-1,4-endoglucanases. Low activities were obtained from cotton and filter paper even though they are cellulosic materials probably due to non-pretreatment of the substrate. Filter paper is often the substrate used to test for exoglucanase activity. The partially purified endoglucanase does not have the capability to cleave 1,4-β-D-xylosidic linkages in xylan, thus the low activity obtained.
Enzymatic hydrolysis of cellulosic materials to produce glucose is predominantly carried out by mesophilic fungi, e.g. Trichoderma, Penicillium and Aspergillus or using cellulolytic enzymes active at mesophilic conditions and neutral pH. A major disadvantage for industrial applications is that most mesophilic cellulases either lack enzymatic activities or are unstable at high temperatures. The maximum activity for most fungal cellulases and β-glucosidases occur at 50±5 °C and pH 4.5 - 5. Usually, they lose about 60% of their activity in the temperature range 50–60 °C and almost completely lose activity at 80 °C due to disorganization of their three-dimensional structures followed by an irreversible denaturation (Verardi et al. 2012). To compete with results from acid hydrolysis, more efficient degradation of the lignocellulosic materials at higher temperature and dilute acid conditions is needed.
The expression of the recombinant thermotolerant acid-stable endoglucanase protein by the transformant Escherichia coli BL21-endoglucanase grown at 37 °C fulfills the ultimate objective of this research. The thermo-acid stable endoglucanase can be used in a supplementary hydrolysis step to further hydrolyze the lignocellulosic materials that were previously treated under high temperature-dilute acid conditions, thereby enhancing the release of more glucose sugars. The temperature of the hydrolysis system does not need to be cooled down to mesophilic (~30–40 °C) levels, thus savings in steam generation and shorter processing time can be achieved. Likewise, a decrease in chemicals and water used for washings are achieved since the pH of the hydrolysis bath need not be adjusted to almost neutral (~pH 6–7) before adding the usually used mesophilic hydrolytic enzyme. The product of the hydrolysis is glucose which can be used in bioethanol production.
Compared to acid hydrolysis, enzymatic approach is promising because it can achieve high sugar (e.g. glucose, xylose) yields, eliminate the need for large quantities of chemicals to lower the pH to almost neutral, decrease the volume of water used for washings, minimize the formation of inhibitory by-products during dilute acid hydrolysis and decrease the over-all processing time.
Conclusion
The culture independent PCR (ciPCR) approach was proven effective in this study with the successful isolation of the endoglucanase gene using the extracted DNA from the pellicle of the mixed culture obtained in the experimental set-up. This is a promising alternative to the isolation of industrially important thermo-acid stable enzymes from selected microorganisms which would require fermentation under high temperature and acidic conditions for growth and enzyme production. The endoglucanase gene successfully amplified from the genomic DNA extracted from a mixed culture grown directly from the inoculation of Brock-CMcellulose (1%) broth with mudspring water from Mt. Makiling, Laguna, Philippines had very high % identity with the glycosyl hydrolase, sso1354 (CelA1; Q97YG7) cloned from a pure culture of Saccharolobus solfataricus. The cloned endoglucanase gene was expressed in a mesophilic E. coli BL21 as expression host. The Mt. Makiling endoglucanase, MM-Engl was thermotolerant up to 80 °C and acid stable up to pH 3. The thermo-acid stable endoglucanase can be used in a supplementary hydrolysis step to further hydrolyze the lignocellulosic materials that were previously treated under high temperature-dilute acid conditions, thereby enhancing the release of more glucose sugars.
References
Akiba S, Kimura Y, Yamamoto K, Kumagai H (1995) Purification and characterization of a protease-resistant cellulase from Aspergillus niger. J Ferment Bioeng 79:125–130. https://doi.org/10.1016/0922-338X(95)94078-6
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Bach HJ, Hartmann A, Schloter M, Munch JC (2001) PCR primers and functional probes for amplification and detection of bacterial genes for extracellular peptidases in single strains and in soil. J Microbiol Methods 44:173–182. https://doi.org/10.1016/S0167-7012(00)00239-6
Bista S (2011) Cloning and Characterization of a novel thermostable endoglucanase from Caldicellulosiruptor bescii in Heterologous Host Escherichia coli. Faculty of Science and Environmental Engineering, Department of Chemistry and Bioengineering, Tampere University of Technology, Tampere, Finland. Unpublished Master of Science thesis. https://www.URN.fi/URN:NBN:fi:tty-2011091514803; https://dspace.cc.tut.fi/dpub/bitstream/handle/123456789/20655/bista.pdf?sequence=6&isAllowed=y
Bok JD, Yernool DA, Eveleigh DE (1998) Purification, characterization, and molecular analysis of thermostable cellulases CelA and CelB from Thermotoga neapolitana. Appl Environ Microbiol 64:4774–4781
Brock TD, Brock KM, Belly RT, Weiss RL (1972) Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol 84:54–68. https://doi.org/10.1007/BF00408082
Brouns SJJ, Ettema TJG, Stedman KM, Walther J, Smidt H, Snijders APL, Young MJ, Bernander R, Wright PC, Siebers B, van der Oost J (2005) The hyperthermophilic archaeon Sulfolobus: from exploration to exploitation. In: Inskeep WP, McDermott TR (eds) Geothermal biology and geochemistry in Yellowstone National park. Thermal Biology Institute, Montana State University, Bozeman, pp 261–276
Bundac AE, Carreon VR, Fernandez A (1976) Report of the thermal and geochemical survey of the geothermal areas in Los Banos and vicinity. Quezon City. Cited in Lantican, N.B., Diaz, M.G.Q., Cantera, J.J.L., de los Reyes, F.L. and Raymundo, A.K. (2011) Microbial community of a volcanic mudspring in the Philippines as revealed by 16S rDNA sequence analysis and fluorescence in situ hybridization. World J Microbiol Biotechnol 27:859–867. https://doi.org/10.1007/s11274-010-0528-y
Cerqueira VS, Hollenbach EB, Maboni F et al (2011) Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour Technol 102:11003–11010. https://doi.org/10.1016/j.biortech.2011.09.074
Costa GL, Grafsky A, Weiner MP (1994) Cloning and analysis of PCR-generated DNA fragments. Genome Res 3:338–345. https://doi.org/10.1101/gr.3.6.338
Cucurachi M, Busconi M, Marudelli M et al (2013) Direct amplification of new cellulase genes from woodland soil purified DNA. Mol Biol Rep 40:4317–4325. https://doi.org/10.1007/s11033-013-2519-1
Dave BR, Sudhir AP, Subramanian RB (2015) Purification and properties of an endoglucanase from Thermoascus aurantiacus. Biotechnol Rep 6:85–90. https://doi.org/10.1016/j.btre.2014.11.004
Dawson SC, Pace NR (2002) Novel kingdom-level eukaryotic diversity in anoxic environments. Proc Natl Acad Sci USA 99:8324–8329. https://doi.org/10.1073/pnas.062169599
DeLong EF, Pace NR (2001) Environmental diversity of bacteria and archaea. Syst Biol 50:470–478. https://doi.org/10.1080/106351501750435040
Dong D, Yan A, Liu H, Zhang X, Xu Y (2006) Removal of humic substances from soil DNA using aluminium sulfate. J Microbiol Methods 66:217–222. https://doi.org/10.1016/j.mimet.2005.11.010
Dorobantu LS, Yeung AKC, Foght JM, Gray MR (2004) Stabilization of oil-water emulsions by hydrophobic bacteria. Appl Environ Microbiol 70:6333–6336. https://doi.org/10.1128/AEM.70.10.6333-6336.2004
Duan XY, Liu SY, Zhang WC et al (2004) Volumetric productivity improvement for endoglucanase of Trichoderma pseudokoingii S-38. J Appl Microbiol 96:772–776. https://doi.org/10.1111/j.1365-2672.2004.02204.x
Edwards IP, Upchurch RA, Zak DR (2008) Isolation of fungal cellobiohydrolase I genes from sporocarps and forest soils by PCR. Appl Environ Microbiol 74:3481–3489. https://doi.org/10.1128/AEM.02893-07
Girfoglio M, Rossi M, Cannio R (2012) Cellulose degradation by Sulfolobus solfataricus requires a cell-anchored endo-β-1-4-glucanase. J Bacteriol 194:5091–5100. https://doi.org/10.1128/JB.00672-12
Grunden AM (2011) Grunden laboratory manual. Department of Plant and Microbial Biology, North Carolina State University (NCSU), Raleigh
Handelsman J (2004) Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 68:669–685. https://doi.org/10.1128/MMBR.68.4.669-685.2004
Handelsman J, Smalla K (2003) Techniques: Conversations with the silent majority. Curr Opin Microbiol 6:271–273. https://doi.org/10.1016/S1369-5274(03)00062-6
Handelsman J, Liles M, Mann D et al (2002) Cloning the metagenome: culture-independent access to the diversity and functions of the uncultivated microbial world. Methods Microbiol 33:241–255. https://doi.org/10.1016/S0580-9517(02)33014-9
Huang Y, Krauss G, Cottaz S, Driguez H, Lipps G (2005) A highly acid-stable and thermostable endo-β-glucanase from the thermoacidophilic archaeon Sulfolobus solfataricus. Biochem J 385:581–588. https://doi.org/10.1042/BJ20041388
Hugenholtz P, Pace NR (1996) Identifying microbial diversity in the natural environment: a molecular phylogenetic approach. Trends Biotechnol 14:190–197. https://doi.org/10.1016/0167-7799(96)10025-1
Jacobsen J, Lydolph M, Lange L (2005) Culture independent PCR: an alternative enzyme discovery strategy. J Microbiol Methods 60:63–71. https://doi.org/10.1016/j.mimet.2004.08.013
Karnchanatat A, Petsom A, Sangvanich P et al (2008) A novel thermostable endoglucanase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. Enzyme Microb Technol 42:404–413. https://doi.org/10.1016/j.enzmictec.2007.11.009
Kikani BA, Shukla RJ, Singh SP (2014) Biocatalytic potential of thermophilic bacteria and actinomycetes. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol 2:1000–1006
Kowalewski E, Stensen JÅ, Gilje E, et al (2004) Interfacial tension measurements in an oil/water/bacteria system by LASER-light scattering. In: International symposium of the society of core analysts. Abu Dhabi, UAE 5–9 October
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lantican NB, Diaz MGQ, Cantera JJL et al (2011) Microbial community of a volcanic mudspring in the Philippines as revealed by 16S rDNA sequence analysis and fluorescence in situ hybridization. World J Microbiol Biotechnol 27:859–867. https://doi.org/10.1007/s11274-010-0528-y
Liu S, Duan X, Lu X, Gao P (2006) A novel thermophilic endoglucanase from a mesophilic fungus Fusarium oxysporum. Chin Sci Bull 51:191–197. https://doi.org/10.1007/s11434-005-1343-y
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lucas R, Robles A, García MT et al (2001) Production, purification, and properties of an endoglucanase produced by the Hyphomycete Chalara (Syn. Thielaviopsis) paradoxa CH32. J Agric Food Chem 49:79–85. https://doi.org/10.1021/jf000916p
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York. https://doi.org/10.1002/jobm.19840240107
Mawadza C, Hatti-Kaul R, Zvauya R, Mattiasson B (2000) Purification and characterization of cellulases produced by two Bacillus strains. J Biotechnol 83:177–187. https://doi.org/10.1016/S0168-1656(00)00305-9
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Mukred AM, Hamid AA, Hamzah A, Wan Yusoff WM (2008) Development of three bacteria consortium for the bioremediation of crude petroleum-oil in contaminated water. Online J Biol Sci 8(4):73–79
Niederberger TD, Götz DK, McDonald IR et al (2006) Ignisphaera aggregans gen. nov., sp. nov., a novel hyperthermophilic crenarchaeote isolated from hot springs in Rotorua and Tokaanu, New Zealand. Int J Syst Evol Microbiol 56:965–971. https://doi.org/10.1099/ijs.0.63899-0
Oles D, Houben G (1998) Greigite (Fe3S4) in an acid mudpool at Makiling volcano, the Philippines. J Asian Earth Sci 16:513–517. https://doi.org/10.1016/S0743-9547(98)00029-4
Pace NR (1997) A molecular view of microbial diversity and the biosphere. Science 276:734–740. https://doi.org/10.1126/science.276.5313.734
Sadhu S, Saha P, Sen SK et al (2013) Production, purification and characterization of a novel thermotolerant endoglucanase (CMcellulase) from Bacillus strain isolated from cow dung. Springerplus 2:10. https://doi.org/10.1186/2193-1801-2-10
Saha BC (2004) Production, purification and properties of endoglucanase from a newly isolated strain of Mucor circinelloides. Process Biochem 39:1871–1876. https://doi.org/10.1016/j.procbio.2003.09.013
Sakai HD, Kurosawa N (2018) Saccharolobus caldissimus gen. nov., sp. nov., a facultatively anaerobic iron-reducing hyperthermophilic archaeon isolated from an acidic terrestrial hot spring, and reclassification of Sulfolobus solfataricus as Saccharolobus solfataricus comb. nov. Int J Syst Evol Microbiol 68:1271–1278. https://doi.org/10.1099/ijsem.0.002665
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Simon C, Daniel R (2009) Achievements and new knowledge unraveled by metagenomic approaches. Appl Microbiol Biotechnol 85:265–276. https://doi.org/10.1007/s00253-009-2233-z
Singh S, Shukla L, Khare S, Nain L (2011) Detection and characterization of new thermostable endoglucanase from Aspergillus awamori strain F 18. J Mycol Pl Pathol 41:97–103
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234. https://doi.org/10.1016/j.pep.2005.01.016
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Tran AV, Chambers RP (1986) Ethanol fermentation of red oak prehydrolysate by the yeast Pichia stipitis CBS 5776. Enzyme Microb Technol 8:439–444. https://doi.org/10.1016/0141-0229(86)90154-7
Verardi A, De Bari I, Ricca E, Calabro V (2012) Hydrolysis of lignocellulosic biomass: current status of processes and technologies and future perspectives. In: Lima, M.A.P., Natalense, A.P.P. (Eds.), Bioethanol. InTech, Rijeka, pp 95–122. ISBN:978-953-51-0008-9
Voget S, Leggewie C, Uesbeck A et al (2003) Prospecting for novel biocatalysts in a soil metagenome. Appl Environ Microbiol 69:6235–6242. https://doi.org/10.1128/aem.69.10.6235-6242.2003
Wang F, Li F, Chen G, Liu W (2009) Isolation and characterization of novel cellulase genes from uncultured microorganisms in different environmental niches. Microbiol Res 164:650–657. https://doi.org/10.1016/j.micres.2008.12.002
Yin LJ, Lin HH, Xiao ZR (2010) Purification and characterization of a cellulase from Bacillus subtilis YJ1. J Mar Sci Technol 18:466–471
Acknowledgements
This research was supported through the ASTHRD Program of the Department of Science and Technology (DOST) of the Philippines (Grant No. 1), a Research Grant from the Fulbright-Philippines Agriculture Scholarship program for Doctoral Dissertation (Grant No. 2), a Research Grant from the Office of the Vice President for Academic Affairs-Emerging Inter-Disciplinary Research Program (OVPAA-EIDR), University of the Philippines-Diliman (Grant No. 3) and BIOTECH-UPLB, University of the Philippines Los Banos. The support of the Microbiology department of North Carolina State University (NCSU), NC, USA, specially the Grunden laboratory and staff are hereby acknowledged. Special thanks to Mr. Bonie Datul for his help preparing the References section.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tambalo, R.D., Raymundo, A.K. & Grunden, A.M. Thermostable endoglucanase gene derived by amplification from the genomic DNA of a cellulose-enriched mixed culture from mudspring water of Mt. Makiling, Laguna, Philippines. World J Microbiol Biotechnol 36, 51 (2020). https://doi.org/10.1007/s11274-020-02825-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02825-2