Abstract
The research paper presents the impact of the scandium additive and various conditions of the heat treatment on copper mechanical, electrical and heat resistance properties. The performed research works included manufacturing of CuSc0.15 and CuSc0.3 alloys through metallurgical synthesis with the use of induction furnace and following crystallization in graphite crucibles at ambient temperature. Additionally, a CuZr0.15 alloy was produced as a reference material for previously synthesized Cu–Sc alloys. During research, the selection of heat treatment for the produced materials was conducted in order to obtain the highest mechanical–electrical properties ratio. Materials obtained in such a way were next subjected to thermal resistance tests. Parameters of thermal resistance test included temperatures from the range of 200–700 °C and 1 h of annealing time. The research has shown that CuSc0.15 and CuSc0.3 alloys have higher heat resistance after their precipitation hardening compared to the Cu–Zr alloy. The paper also presents microstructural research of the produced materials, which showed that alloying elements precipitates are mainly localized at the grain boundaries of the material structure.
Similar content being viewed by others
1 Introduction
Due to its high electrical properties, copper and its alloys are widely used in every application where various electrical connections in the form of cables, wires and electrical joints exist. A special case includes elements in which a high electrical conductivity of the material is required at the same time as its high mechanical properties at elevated temperatures. These are, among others electrical connectors, lead frames, switches, switch blade jaws, commutators for power transmitters, basses for power transmitters, rectifiers, soldering and welding tips, circuit breakers, spring contacts [1] and electronic circuits [2]. Considering pure copper, in order to obtain high mechanical properties, a strain hardening effect needs to be achieved with the use of cold forming processes [3] in the case of precipitation hardening copper alloys; a heat treatment or thermo-mechanical treatment is often performed, which strengthens the structure of the material due to dispersion hardening phenomena. However, in order for the heat treatment process to be conducted, the alloy must meet two conditions which are a substantial solubility of one component in the other at elevated temperature and solubility limit of alloying element that decreases along with temperature drop allowing to supersaturate the solution during rapid cooling of the alloy [4]. Confirmation of above-mentioned phenomena can be done by investigation of given alloy phase diagram which nowadays is often obtained with the use of CALPHAD method as shown in [5] where authors calculated Cu–Sc phase diagram system. An example of the copper alloys which can be subjected to the thermo-mechanical treatment is Cu–Zr alloy. Zirconium is a commonly used alloy additive which in case of copper allows to increase its heat resistance, mechanical and exploitational properties [6]. Furthermore, the addition of Zr in the amount of 0.1–0.2 wt% provides a high increase in heat resistance with no significant decrease in electrical properties which is difficult to achieve in case of other alloying elements such as Cr, Mg, Ag, Ni or Ti. These alloys are characterized with decent formability during cold working like open die forging, extrusion or rolling. Cu–Zr alloys with 0.05–0.15 wt% of Zr are mainly used in automotive industry as switches and relays, contacts, connectors, terminals and parts of hybrid cars. Additionally, zirconium copper is commonly used in the electrotechnical industry as electronic circuits, electrical connectors, lead frames, switches, switch blade jaws, commutators for power, transmitters, basses for power transmitters, rectifiers, soldering and welding tips, circuit breakers and high-temperature applications.
One of the most popular commercialized alloys used for the applications mentioned above is CuZr0.15. Depending on the structural state which is associated with the manufacturing technology, its functional properties may vary, i.e., UTS 255–490 MPa, YS 60–455 MPa, elongation 35–2%, hardness 70–110 HV10, and electrical conductivity is approx. 55 MS/m. The comprehensive properties data of the commercial CuZr0.15 alloy in different tempers are given in Table 1.
CuZr0.15 alloy manufactured with traditional metallurgical synthesis followed by a heat treatment process and cold metal forming is characterized by high heat resistance in 1-h test up to 400 °C as shown in Fig. 1. Higher increase in the heat resistance of Cu–Zr alloys is possible through higher amount of Zr addition (up to 0.8 wt%) which was proved by authors in [7] by the use of powder metallurgy method during production of the alloy which enables to rise the softening temperature even up 600 °C. This however significantly rises the final cost of the finished product, due to the used technology. Examples of the softening characteristics for various Zr content in copper are shown in Fig. 2.
RB hardness measured at 20 °C as a function of annealing temperature (1-h test) of various as-extruded (powder metallurgy) Cu–Zr alloys, i.e., ZA-2—CuZr0.2, ZA-3—CuZr0.37, ZA-8—CuZr0.8, ZAC-1—CuCr0.32Zr0.1)—[7]
The advantageous effect of zirconium additive is also noticeable in case of Cu–Cr alloys. Cu–Cr–Zr alloys with usually 0.6–1 wt% of Cr and 0.04–0.1 wt% of Zr are characterized by high mechanical properties shown in [7,8,9] and improved wear properties shown in [10] as well as high heat resistance of up to 500 °C [7]. However, due to the content of alloy additive above 1 wt%, the electrical conductivity of this group of alloys is approx. 50 MS/m and lower which makes it necessary to increase the cross section of the final products in their electrical applications. In [9] and [11], authors showed that it is possible to increase both electrical and mechanical properties through thermal and thermo-mechanical processing of Cu–Cr–Zr alloys. In some cases, Zr was also found to increase electrical conductivity and refine structure of Cu–Ni–Si alloy as shown in [12].
According to one’s own analysis of the physicochemical properties of various elements, another alloy additive that favorably affects the copper softening temperature while maintaining its high electrical properties is scandium. However, during the literature analysis, it has turned out that research works done so far have not precisely defined the impact of the scandium additive to copper alloys on its mechanical, electrical, structural or heat resistance properties. Two of the few publications available in this field of expertise are works [13, 14] in which it has been proven that the scandium additive in the amount of 0.2 wt% and 0.4 wt% causes an increase in the hardness of the CuSc0.2 alloy from 60 HV10 to 70 HV10 and of CuSc0.4 alloy from 65 HV10 to 95 HV10 as a result of their artificial aging for 7 h at 400 °C. Research concerning other Cu–Sc alloys properties has not yet been published which limits the knowledge of the impact of Sc alloy addition on copper functional properties during the metal forming, heat treatment, heat resistance, etc., per analogy; it is well-known that addition of scandium to aluminum increases its mechanical and technological properties. Research results presented in [15] show increase in yield strength after addition of Sc into Al–Cu alloy. In [16], authors presented that addition of scandium in SSA018 alloy also allows to improve yield strength and the ultimate strength due to precipitation of the GP II zones and η′ phase particles. As shown in [17], mechanical properties of Sc-modified 7075 aluminum alloy can be further improved by the use of equal-channel angular pressing technology followed by the low-temperature aging process. In [18], authors found out that addition of Sc and Zr to Al–Cu–Mg alloy allows to increase corrosion resistance due to the change in cross-section morphology of tested samples.
As demonstrated by one’s own pilot research, even a small scandium additive to copper alloys effectively contributes to the increase in the copper softening temperature while maintaining its initial high electrical properties. Therefore, further research work was conducted on the characterization of exploitational and technological properties of copper alloys with scandium additive up to 0.5 wt%. This research paper focuses on the study on manufacturing of CuSc0.15 and CuSc0.3 alloys and on the development of heat treatment process parameters optimized in a way to maximize mechanical and electrical properties of analyzed materials. In addition, the results of heat resistance tests of manufactured alloys in the temperature range of 200–700 °C in 1-h annealing tests are presented. The obtained test results were next compared with the CuZr0.15 reference material. In nowadays manufacturing industry, the Cu–Sc alloys are not commonly used because of the lack of both literature information and the possible uses of these alloys. They might potentially replace Cu–Zr alloys among the applications which require high set of mechanical and electrical properties and high resistance to softening.
2 Materials and methods
Based on the phase diagram, three intermetallic compounds were found in Cu–Sc alloy, i.e., Cu4Sc, Cu2Sc and CuSc with the scandium content of 15, 25 and 42 wt%, respectively. Maximum solubility of scandium in copper was determined to be about 0.5 wt% at the temperature of 1130 K (Fig. 3). As shown in the literature, in the case of alloys with scandium amount under 0.5 wt% in copper, during the artificial annealing of supersaturated solution, the dispersion phase of Cu4Sc is precipitated which causes the hardening of the material [5, 19]. Due to above, two types of Cu–Sc alloys containing 0.15 wt% and 0.3 wt% of scandium were selected for further experimental research as well as the reference alloy Cu–Zr containing 0.15 wt% of zirconium.
Cu–Sc phase diagram [20]
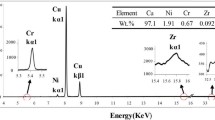
All selected materials were next manufactured with the use of traditional metallurgical synthesis process in ϕ40 × 100-mm graphite crucibles using an induction furnace. After melting, the molten metal homogenization processes was carried out at 1350 ± 10 °C temperature for 30 min in a reducing atmosphere obtained through flake graphite addition on molten metal surface. Afterward, the crucibles were taken out from the induction furnace, which further resulted in their crystallization at ambient temperature within 2 min. Figure 4 shows all three types of obtained ingots prepared for further analysis. Table 2 presents the chemical composition of obtained casts produced with the X-ray fluorescence method.
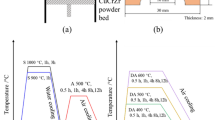
The obtained casts with diameter of 40 mm were next cut in their cross section into samples with a thickness of 5 mm and subjected to heat treatment research carried out at 450 °C for up to 25 h for as-cast temper. After that, heat treatment samples were analyzed for their hardness and electrical conductivity. After determination of the best artificial aging conditions for tested materials in terms of their highest possible electrical conductivity and hardness, heat-treated materials were subjected to a 1-h thermal resistance tests (softening curve) in the temperature range of 200–700 °C.
As part of the research program, an open die hot forging process was conducted on the samples with diameter of 14 mm and the height of 10 mm which were machined from obtained ingots. At first during these tests, CuSc0.15, CuSc0.3 and CuZr0.15 samples were annealed at 950 °C for 3 h and hot forged (from 950 °C) to the final height of 3 mm which gives a total strain of approx. ɛ = 70%. After the forging process, all samples were cooled down using water in order to obtain ambient temperature. On all forgings, the selection of artificial aging parameters at 450 °C up to 5 h was conducted. Per analogy to the initial heat resistance research results made on the as-cast alloys the range of temperatures of between 300 °C and 700 °C was selected.
For all obtained samples, measurements of electrical conductivity were conducted using the SigmaTest 2.069 device, while the hardness was measured using the Vickers method using the Tukon2500 device. Also as-cast materials were also subjected to microstructural analyses which were performed with the use of scanning electron microscope Hitachi SU-70 in order to perform qualitative and quantitative analysis of the precipitations.
3 Results
Firstly, microstructural analysis of the obtained copper alloy casts (CuSc0.15, CuSc0.3 and the reference alloy CuZr0.15) was carried out in which results are presented in Figs. 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16. The microstructural SEM observation included EDS mapping in order to determine the distribution of given alloying addition in copper.
Figures 17, 18 and 19 present the artificial aging characteristics of the CuSc0.15, CuSc0.3 and CuZr0.15 alloys in their as-cast state at 450 °C, as hardness and electrical conductivity characteristics versus aging time. Figures 20, 21 and 22 show the same set of results for the materials after hot open die forging process. In both cases, temperature of 450 °C has been chosen as favorable due to previously conducted pilot studies.
Figure 23 shows the results of the CuSc0.15, CuSc0.3 and CuZr0.15 alloys softening tests in the temperature range from 200 to 700 °C, while Fig. 24 shows the softening curves of these materials after hot metal forming and following artificial aging at the temperature range of 300 °C to 700 °C.
4 Discussion
The microstructural analyses of the casts showed that in the case of the CuSc0.15 alloy (Fig. 3), the scandium is located on the grain boundaries creating oval shapes (eutectics) in the material structure. EDS analysis showed that only a small part of the Sc additive is located in the solution. From the microstructural analysis of the CuSc0.3 alloy (Fig. 4), a similar relation can be observed as in the case of the alloy with a lower content of scandium. The scandium is also located at the grain boundaries of the material, as shown by the EDS analysis. In the case of the CuZr0.15 alloy, the microstructural analysis (Fig. 5) shows similarities to scandium. Zirconium is located mainly at the grain boundaries, with small amount remaining in the solution. The analysis of the precipitates indicates that both CuSc0.15 and CuSc0.3 as well as CuZr0.15 casts in the selected structural state (as-cast) show multiphase structure with a visible eutectics. Morphology of these precipitates, their size (5–10 µm), shape and the fact that they are mainly located at the grain boundaries indicates that these are primary precipitates resulting from free crystallization of the ingots. Such precipitates in the structure of the alloy are unfavorable both from the point of view of susceptibility to artificial aging and the final functional properties of the alloy. Due to the size of these precipitates, they will be difficult to dissolve in the solid state during the high-temperature homogenization process. In order to reduce the occurrence of large primary precipitates in the analyzed alloys, it is advantageous to supercool the alloys during crystallization and quickly cool down to temperatures below 400 °C, where the precipitation processes are rapidly slowed down. The alloys produced in this way will certainly have a more favorable structure from the point of view of their final properties obtained through artificial aging.
Heat treatment analysis of copper alloys covered by this article show that the CuSc0.15 alloy (Fig. 17) requires an artificial aging at 450 °C for 15 h to obtain the highest set of mechanical and electrical properties. The thermally activated precipitation processes resulted in the increase in the hardness from 43 HV to 62 HV, while the electrical conductivity increases from 43 to 52 MS/m. In the case of the CuSc0.3 alloy (Fig. 18), the highest set of properties is obtained after 20 h of artificial aging. The hardness of the alloy after 20 h increases from 55 HV to 70 HV, while the electrical conductivity increases from 38 to 50 MS/m. Analysis of the properties and characteristics of the CuZr0.15 alloy shows that aging response of this alloy is much quicker than for the alloy with the addition of scandium. CuZr0.15 alloy (Fig. 19) achieves the highest mechanical and electrical properties after 4 h, with hardness at 78 HV, and electrical conductivity of 55 MS/m. Figures 25 and 26 present comprehensive data regarding variations in hardness and electrical conductivity during artificial aging of the analyzed alloys, i.e., CuSc0.15, CuSc0.3 and the reference alloy CuZr0.15. The gradual overaging of the CuZr0.15 alloy resulting in the decrease in mechanical properties begins just after 4 h which is almost a quarter of the time needed to achieve the highest mechanical properties for the CuSc0.15 alloy and only one fifth of the time needed for the CuSc0.3 alloy. One point of view is that the shorter artificial aging time for the CuZr0.15 alloy at 450 °C is an advantage in technological terms; however, it directly indicates that the 450 °C temperature is dangerous from the point of view of possible overaging of the alloy which may transfer to a much lower heat resistance than CuSc0.15 and CuSc0.3 alloys. Even though CuZr0.15 alloy after 4 h of annealing had almost two times higher hardness values than Cu–Sc alloys during longer heat treatment (in 20 h), CuZr0.15 has the same hardness value as CuSc0.3.
Based on the analysis of the mechanical properties of CuSc0.15, CuSc0.3 and CuZr0.15 alloys at various times of the artificial aging process, i.e., for 1, 4, 10, 15, 20 and 25 h as shown in Table 3, conclusions can be made that CuSc0.15 and CuSc0.3 achieve the highest values of the mechanical properties at 15 h and 20 h of heat treatment, respectively, and CuZr0.15 alloy at 4 h may be made. Such a significant difference in aging time needed to obtain the maximum values of mechanical properties, as shown by the research, proves that CuSc0.15 and CuSc0.3 alloys have a much higher heat resistance than CuZr0.15 in terms of 1-h annealing process at 200–700 °C.
Considering the alloys after the hot open die forging process, it is visible that the precipitation hardening occurs much quicker than in the case of the ingots. Figures 27 and 28 present the comprehensive hardness and electrical conductivity characteristics for tested materials in the function of their artificial aging time.
Analyzing the results of the research on the artificial aging of the alloys after the hot metal forming can be concluded that these alloys reach the extremum of the mechanical properties after 45 min of aging at 450 °C. After this time, the hardness gradually decreases for every analyzed alloy. The hardness of CuSc0.15 alloy increases from the value of 87 HV after forging to 95 HV after 45 min of aging, whereas the electrical conductivity increases from 38 to 44.3 MS/m. Considering the CuSc0.3 alloy, the hardness after 45 min of aging increases from 90 HV to 109 HV, and the electrical conductivity increases from 30 to 44 MS/m. The highest amount of scandium additive in the alloy provides higher value of hardness (by 14 HV) with the same electrical conductivity after 45 min of aging. Analysis of CuZr0.15 alloy properties after forging and aging show that the hardness of this alloy increases from 84.5 HV to 89 HV and the electrical conductivity from 45 to 53.4 MS/m. In conclusion, the conducted research works show that the alloys with zirconium additive show the lowest susceptibility to hardening through hot metal forming and aging than the alloys with scandium additive. Table 4 shows the changes in hardness of the tested materials after various times of artificial aging at 450 °C.
On the basis of the conducted softening tests of the materials after heat treatment under the indicated conditions (CuSc0.15—15 h, CuSc0.3—20 h, CuZr0.15—4 h), it can be concluded that copper with the addition of scandium has much higher softening temperature in a 1-h annealing process in the temperature range from 200 to 700 °C than the copper–zirconium alloy. Figure 23 presents the softening curves of CuSc0.15, CuSc0.3 and CuZr0.15 alloys in the temperature range of 200 °C to 700 °C during 1 h of heat treatment. Figure 24 shows the softening curves of the materials after hot metal forming and artificial aging for temperature range from 300 to 700 °C during 1 h of heat treatment process. The percentage change in the hardness of CuSc0.15, CuSc0.3 and CuZr0.15 alloys as a result of softening being presented in Figs. 29 and 30 was determined according to the following rule:
where Hd—percentage hardness decrease in the materials in relation to the initial value, Hx°C—hardness of the material at the given annealing temperature (200–700 °C) and H20°C—hardness of the material at 20 °C.
The CuSc0.15 alloy maintains a stable hardness up to 450 °C, while the CuSc0.3 alloy up to 500 °C. In the case of CuZr0.15 alloy, although initially (after artificial aging) it has the highest hardness, when subjected to elevated temperature, the gradual decrease in its hardness occurs even at the temperatures above 250 °C. Table 5 presents the values of the hardness percentage decrease in CuSc0.15, CuSc0.3 and CuZr0.15 alloys after 1-h annealing process in 300 °C, 400 °C and 500 °C according to which CuSc0.15 and CuSc0.3 alloys mechanical properties at 300 °C do not decrease. Meanwhile at 300 °C, CuZr0.15 hardness decreases by 5%. At 500 °C, CuSc0.3 hardness decreases by less than 2%, while CuSc0.15 decreases by 5%. CuZr0.15 alloy at 500 °C decreases its hardness values by 16.5%.
The conducted research results presented in Table 6 show that in the case of CuSc0.15 alloy, the mechanical properties maintain stability up to 500 °C of artificial aging where the percentage decrease in hardness is equal to 1.8% in reference to the starting material. The same situation is visible considering CuSc0.3 alloy where the value of percentage decrease in hardness is only 0.6% at 500 °C. CuZr0.15 alloy maintains its stability of mechanical properties up to 450 °C of artificial aging. After aging at 500 °C, the percentage decrease in hardness is equal to 3.5% which is twice the value of the decrease in CuSc0.15 alloy.
5 Conclusions
Based on the results of the research, the following conclusions were made:
-
1.
Free crystallization and slow self-cooling of copper–scandium alloys results in the formation of large primary precipitates of Cu–Sc in its structure, which because of their shape, size and location on the grain boundaries are disadvantageous from the point of view of the final properties obtained as a result of artificial aging.
-
2.
Precipitates with a high amount of Sc, with the size of 5–10 µm and multiphase structure will be very difficult to dissolve in the Cu–Sc matrix during the homogenization process which will result in a limited susceptibility to the artificial aging.
-
3.
Achieving the highest properties of CuSc0.15 and CuSc0.3 alloys as a result of artificial aging at 450 °C requires a long exposure time, i.e., 15 and 20 h, respectively, while for the reference alloy, i.e., CuZr0.15, it is only 4 h.
-
4.
Considering the artificial aging of CuSc0.15, CuSc0.3 and CuZr0.15 alloys after hot metal forming their extremum of hardness is reached after 45 min of heat treatment.
-
5.
The addition of scandium in the copper alloy causes a favorable increase in the softening temperature of Cu–Sc alloys. Scandium addition in the amount of 0.15% wt. in copper and artificial aging of the as-cast material directly after its crystallization provides thermal stability of the alloy up to a temperature of 450 °C after 1-h annealing, and an increase in the content of the scandium to 0.3% wt. causes a further increase in the thermal stability of the alloy by 50 °C, i.e., up to 500 °C (for 1 h). A cast containing 0.15% wt. of Zr produced in an analogous manner has thermal stability up to 250 °C. On the basis of the conducted research, it was shown that the scandium additive of 0.15–0.3 wt% to copper has a positive impact on the increase in the softening temperature to 450 °C and 500 °C, respectively, during 1 h of annealing.
-
6.
The research on the heat resistance of the materials after how metal forming and heat treatment during 1 h of annealing proved that the mechanical properties of Cu–Sc alloys maintain stable up to 500 °C, whereas CuZr0.15 alloy maintains the stability of its mechanical properties only up to 450 °C.
-
7.
The conducted research has shown that addition of scandium can be an alternative to a typically used zirconium alloys, favorably affecting their functional properties, in particular increasing mechanical properties and their heat resistance with a slight decrease in the electrical properties of the alloys.
References
Davis JR. ASM specialty handbook: copper and copper alloys. Materials Park: ASM International; 2001.
Liu P, Tong L, Wang J, Shi L, Tang H. Challenges and developments of copper wire bonding technology. Microelectron Reliab. 2012;52:1092–8.
Guo N, Li D, Yu H, Xin R, Zhang Z, Li X, Liu C, Song B, Chai L. Annealing behavior of gradient structured copper and its effect on mechanical properties. Mater Sci Eng A. 2017;702:331–42.
Campell FC. Elements metallurgy and engineering alloys. Materials Park: ASM International; 2018. p. 139–45.
Bo H, Liu LB, Jin ZP. Thermodynamic analysis of Al–Sc, Cu–Sc and Al–Cu–Sc system. J Alloys Compd. 2010;490:318–25.
Zhao Y, Pang T, He J, Tao X, Chen H, Ouyang Y, Du Y. Interdiffusion behaviors and mechanical properties of Cu–Zr system. Calphad. 2018;61:92–7.
Sarin VK, Grant NJ. Cu–Zr and Cu–Cr–Zr alloys produced from rapidly quenched powders. Metall Trans. 1972;3:875–8.
Batra IS, Dey GK, Kulkarni UD, Banerjee S. Microstructure and properties of a Cu–Cr–Zr alloy. J Nucl Mater. 2001;299:91–100.
Wei T, Liming B, Fengcang M, Jiandi D. Effect of Zr on as-cast microstructure and properties of Cu–Cr alloy. Vacuum. 2018;149:238–47.
Tu JP, Qi WX, Yang YZ, Liu F, Zhang JT, Gan GY, Wang NY, Zhang XB, Liu MS. Effect of aging treatment on the electrical sliding wear behavior of Cu–Cr–Zr alloy. Wear. 2002;249:1021–7.
Huang AH, Wang YF, Wang MS, Song LY, Li YS, Gao L, Huang CX, Zhub YT. Optimizing the strength, ductility and electrical conductivity of a Cu–Cr–Zr alloy by rotary swaging and aging treatment. Mater Sci Eng A. 2019;746:211–6.
Wang W, Kang H, Chen Z, Chen Z, Zou C, Li R, Yin G, Wang T. Effects of Cr and Zr additions on microstructure and properties of Cu–Ni–Si alloys. Mater Sci Eng A. 2016;673:378–90.
Zakharov MV, Zakharov AM, Popov OP, Dashevskaya NE. Effect of scandium on the properties of copper and certain copper alloys. Izvest Vuz Tsvetnaya Met. 1970;4:117–21.
Watanabe S, Kleppa OJ. Thermochemistry of alloys of transition metals: part IV. Alloys of copper with scandium, yttrium, lanthanum, and lutetium. Metall Trans B. 1984;15:357–68.
Yang C, Shao D, Zhang P, Gao YH, Zhang JY, Kuang J, Wu K, Liu G, Sun J. The influence of Sc solute partitioning on ductile fracture of Sc-microalloyed Al–Cu alloys. Mater Sci Eng A. 2018;717:113–23.
Senkov ON, Shagiev MR, Senkova SV, Miracle DB. Precipitation of Al3(Sc, Zr) particles in an Al–Zn–Mg–Cu–Sc–Zr alloy during conventional solution heat treatment and its effect on tensile properties. Acta Mater. 2008;56:3723–38.
Kim WJ, Kim JK, Kim HK, Park JW, Jeong YH. Effect of post equal-channel-angular-pressing aging on the modified 7075 Al alloy containing Sc. J Alloys Compd. 2008;450:222–8.
Sun F, Nash GL, Li Q, Liu E, He C, Shi C, Zhao N. Effect of Sc and Zr additions on microstructures and corrosion behavior of Al–Cu–Mg–Sc–Zr alloys. J Mater Sci Technol. 2017;33:1015–22.
Turchanin MA. Phase equilibria and thermodynamics of binary copper systems with 3 d-metals. I. the copper-scandium system. Powder Metall Met Ceram. 2006;45:143–52.
Predel B. Phase equilibria, crystallographic and thermodynamic data of binary alloys. Physical chemistry. 1st ed. Berlin, Heidelberg: Springer; 2016.
ZHC Copper, UNS C15100, H01 Temper, MatWeb, Your Source for Materials Information—www.matweb.com.
ZHC Copper, UNS C15100, H02 Temper, MatWeb, Your Source for Materials Information—www.matweb.com.
ZHC Copper, UNS C15100, H03 Temper, MatWeb, Your Source for Materials Information—www.matweb.com.
ZHC Copper, UNS C15100, H06 Temper, MatWeb, Your Source for Materials Information—www.matweb.com.
ZHC Copper, UNS C15100, H08 Temper, MatWeb, Your Source for Materials Information—www.matweb.com.
Acknowledgements
Funding was provided by Narodowe Centrum Badań i Rozwoju (Grant No. TECHMATSTRATEG1/347960/6/NCBR/2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franczak, K., Kwaśniewski, P., Kiesiewicz, G. et al. Research of mechanical and electrical properties of Cu–Sc and Cu–Zr alloys. Archiv.Civ.Mech.Eng 20, 28 (2020). https://doi.org/10.1007/s43452-020-00035-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-020-00035-z