Abstract
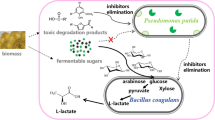
In this study, we constructed a coculture consortium comprising engineered Pseudomonas putida KT2440 and Escherichia coli MG1655. Provision of “related” carbon sources and synthesis of medium-chain-length polyhydroxyalkanoates (mcl-PHAs) were separately assigned to these strains via a modular construction strategy. To avoid growth competition, a preference for the use of a carbon source was constructed. Further, the main intermediate metabolite acetate played an important role in constructing the expected “nutrition supply–detoxification” relationship between these strains. The coculture consortium showed a remarkable increase in the mcl-PHA titer (0.541 g/L) with a glucose–xylose mixture (1:1). Subsequently, the titer of mcl-PHA produced by the coculture consortium when tested with actual lignocellulosic hydrolysate (0.434 g/L) was similar to that achieved with laboratory sugars’ mixture (0.469 g/L). These results indicate a competitive potential of the engineered E. coli–P. putida coculture consortium for mcl-PHA production with lignocellulosic hydrolysate.





Similar content being viewed by others
References
Agnew DE, Pfleger BF (2013) Synthetic biology strategies for synthesizing polyhydroxyalkanoates from unrelated carbon sources. Chem Eng Sci 103:58–67. https://doi.org/10.1016/j.ces.2012.12.023
Ashby RD, Solaiman DKY, Foglia TA, Liu CK (2001) Glucose/lipid mixed substrates as a means of controlling the properties of medium chain length poly(hydroxyalkanoates). Biomacromolecules 2:211–216. https://doi.org/10.1021/bm000098+
Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Braunegg G, Bona R, Koller M (2004) Sustainable polymer production. J Macromol Sci Part D Rev Polym Process 43:1779–1793
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-β -hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6:29–37
Causey TB, Zhou S, Shanmugam KT, Ingram LO (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc Natl Acad Sci USA 100:825–832. https://doi.org/10.1073/pnas.0337684100
Cesario MT, Raposo RS, de Almeida M, van Keulen F, Ferreira BS, da Fonseca MMR (2014) Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol 31:104–113. https://doi.org/10.1016/j.nbt.2013.10.004
Sambrook J (2001) Molecular cloning: a laboratory manual. Anal Biochem 186(1):182–183
Davis R, Kataria R, Cerrone F, Woods T, Kenny S, O’Donovan A, Guzik M, Shaikh H, Duane G, Gupta VK (2013) Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Biores Technol 150:202–209
Escapa IF, del Cerro C, Garcia JL, Prieto MA (2013) The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol 15:93–110. https://doi.org/10.1111/j.1462-2920.2012.02790.x
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microbial Cell Fact 4(1):14. https://doi.org/10.1186/1475-2859-4-14
Gowda V, Shivakumar S (2014) Agrowaste-based polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz Arch Biol Technol 57:55–61. https://doi.org/10.1590/s1516-89132014000100009
Hirakawa H, Takumi-Kobayashi A, Theisen U, Hirata T, Nishino K, Yamaguchi A (2008) AcrS/EnvR represses expression of the acrAB multidrug efflux genes in Escherichia coli. J Bacteriol 190:6276–6279
Jiang X, Ramsay JA, Ramsay BA (2006) Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J Microbiol Methods 67:212–219
Jones JA, Wang X (2018) Use of bacterial co-cultures for the efficient production of chemicals. Curr Opin Biotechnol 53:33–38
Kenny ST, Runic JN, Kaminsky W, Woods T, Babu RP, O’Connor KE (2012) Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl Microbiol Biotechnol 95:623–633. https://doi.org/10.1007/s00253-012-4058-4
Kobayashi H, Fukuoka A (2013) Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem 15:1740–1763. https://doi.org/10.1039/c3gc00060e
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. https://doi.org/10.1016/0378-1119(95)00584-1
Kulkarni SO, Kanekar PP, Jog JP, Sarnaik SS, Nilegaonkar SS (2015) Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int J Biol Macromol 72:784–789. https://doi.org/10.1016/j.ijbiomac.2014.09.028
Le Meur S, Zinn M, Egli T, Thony-Meyer L, Ren Q (2012) Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. Bmc Biotechnol 12(1):53. https://doi.org/10.1186/1472-6750-12-53
Lee GN, Na J (2013) Future of microbial polyesters. Microb Cell Fact 12:54
Lin ZQ, Xu ZB, Li YF, Wang ZW, Chen T, Zhao XM (2014) Metabolic engineering of Escherichia coli for the production of riboflavin. Microbial Cell Fact 13(1):104. https://doi.org/10.1186/s12934-014-0104-5
Liu X, Li XB, Jiang JL, Liu ZN, Qiao B, Li FF, Cheng JS, Sun XC, Yuan YJ, Qiao JJ, Zhao GR (2018) Convergent engineering of syntrophic Escherichia coli coculture for efficient production of glycosides. Metab Eng 47:243–253. https://doi.org/10.1016/j.ymben.2018.03.016
Lowe H, Hobmeier K, Moos M, Kremling A, Pfluger-Grau K (2017) Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB. Biotechnol Biofuels 10(1):190. https://doi.org/10.1186/s13068-017-0875-0
Narayanan A, Kumar VAS, Ramana KV (2014) Production and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus mycoides DFC1 using rice husk hydrolyzate. Waste Biomass Valoriz 5:109–118. https://doi.org/10.1007/s12649-013-9213-3
Nizami AS, Korres NE, Murphy JD (2009) Review of the integrated process for the production of grass biomethane. Environ Sci Technol 43:8496–8508. https://doi.org/10.1021/es901533j
Obruca S, Benesova P, Petrik S, Oborna J, Prikryl R, Marova I (2014) Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem 49:1409–1414. https://doi.org/10.1016/j.procbio.2014.05.013
Pan WY, Perrotta JA, Stipanovic AJ, Nomura CT, Nakas JP (2012) Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J Ind Microbiol Biotechnol 39:459–469. https://doi.org/10.1007/s10295-011-1040-6
Poblete-Castro I, Binger D, Rodrigues A, Becker J, dos Santos V, Wittmann C (2013) In-silico-driven metabolic engineering of Pseudomonas putida for enhanced production of poly-hydroxyalkanoates. Metab Eng 15:113–123. https://doi.org/10.1016/j.ymben.2012.10.004
Pos KM (2009) Drug transport mechanism of the AcrB efflux pump. Biochim Biophys Acta Proteins Proteom 1794:782–793
Prabu CS, Murugesan AG (2010) Effective utilization and management of coir industrial waste for the production of poly-β-hydroxybutyrate (PHB) using the bacterium Azotobacter beijerinickii. Int J Environ Res 4:519–524
Rai R, Keshavarz T, Roether JA, Boccaccini AR, Roy I (2011) Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng R-Rep 72:29–47. https://doi.org/10.1016/j.mser.2010.11.002
Sang YL (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Sawant SS, Salunke BK, Kim BS (2015) Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp LL1. Biores Technol 194:247–255. https://doi.org/10.1016/j.biortech.2015.07.019
Shalin T, Sindhu R, Pandey A, Faraco V, Binod P (2016) Production of poly-3-hydroxybutyrate from mixed culture. Biologia 71(7):736–742
Shin KS, Lee SK (2017) Increasing extracellular free fatty acid production in Escherichia coli by disrupting membrane transport systems. J Agric Food Chem 65(51):11243–11250
Silva JA, Tobella LM, Becerra J, Godoy F, Martínez MA (2007) Biosynthesis of poly-β-hydroxyalkanoate by Brevundimonas vesicularis LMG P-23615 and Sphingopyxis macrogoltabida LMG 17324 using acid-hydrolyzed sawdust as carbon source. J Biosci Bioeng 103:542–546
Silva LF, Taciro MK, Michelin Ramos ME, Carter JM, Pradella JGC, Gomes JGC (2004) Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J Ind Microbiol Biotechnol 31:245–254
Sim SJ, Snell KD, Hogan SA, Stubbe J, Rha C, Sinskey AJ (1997) PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat Biotechnol 15:63–67. https://doi.org/10.1038/nbt0197-63
Griffiths E (1991) Prokaryotic physiology in perspective. Physiology of the Bacterial Cell: a molecular approach by F. C. Neidhart, J. L. Ingraham and M. Schaechter, Sinauer Associates, 1990. £34.95 (xii + 506 pages) ISBN 0 87893 608 4. Trends Biotechnol 9(1):442–443
Song H, Ding MZ, Jia XQ, Ma Q, Yuan YJ (2014) Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev 43:6954–6981. https://doi.org/10.1039/c4cs00114a
Steinbüchel A, Füchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Valappil SP, Rai R, Bucke C, Roy I (2008) Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J Appl Microbiol 104:1624–1635
Wang BQ, Sharma-Shivappa RR, Olson JW, Khan SA (2013) Production of polyhydroxybutyrate (PHB) by Alcaligenes latus using sugarbeet juice. Ind Crops Prod 43:802–811. https://doi.org/10.1016/j.indcrop.2012.08.011
Wang Q, Tappel RC, Zhu CJ, Nomura CT (2012) Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks. Appl Environ Microbiol 78:519–527. https://doi.org/10.1128/aem.07020-11
Wang Q, Zhuang QQ, Liang QF, Qi QS (2013) Polyhydroxyalkanoic acids from structurally-unrelated carbon sources in Escherichia coli. Appl Microbiol Biotechnol 97:3301–3307. https://doi.org/10.1007/s00253-013-4809-x
Wang Y, Yin J, Chen G-Q (2014) Polyhydroxyalkanoates, challenges and opportunities. Curr Opin Biotechnol 30:59–65
Xu P, Li L, Zhang F, Stephanopoulos G, Koffas M (2014) Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci USA 111:11299–11304
Xu P, Qiao K, Ahn WS, Stephanopoulos G (2016) Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc Natl Acad Sci 113:10848–10853
Yang SY, Li SH, Jia XQ (2019) Production of medium chain length polyhydroxyalkanoate from acetate by engineered Pseudomonas putida KT2440. J Ind Microbiol Biotechnol 46:793–800. https://doi.org/10.1007/s10295-019-02159-5
Yu J, Stahl H (2008) Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Biores Technol 99:8042–8048. https://doi.org/10.1016/j.biortech.2008.03.071
Zhang HR, Wang XN (2016) Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng 37:114–121. https://doi.org/10.1016/j.ymben.2016.05.007
Zhou K, Qiao KJ, Edgar S, Stephanopoulos G (2015) Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33(4):377–383
Acknowledgements
The authors are grateful for the kind donation of Escherichia coli MG1655 from Dr. Tao Chen and the plasmid pBBR1MCS-2 from Dr. Yingjin Yuan at Tianjin University. The authors wish to acknowledge the financial support provided by the National Key Research and Development Program of China (Project no. 2018YFA0902100), National Natural Science Foundation of China (No. 21576197), and Tianjin Research Program of Application Foundation and Advanced Technology (No. 18JCYBJC23500).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Yang, S. & Jia, X. Construction of a “nutrition supply–detoxification” coculture consortium for medium-chain-length polyhydroxyalkanoate production with a glucose–xylose mixture. J Ind Microbiol Biotechnol 47, 343–354 (2020). https://doi.org/10.1007/s10295-020-02267-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02267-7