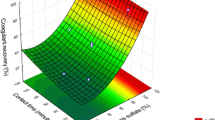
The subject of study was the Safyanovskaya Med company’s acidic underspoil water containing 0.17 g/liter of copper and 1.8 g/liter of zinc (pH 2.8–2.9). The goal was to study and develop a technology of cleaning water of impurities (copper, zinc, aluminum, iron, manganese), accompanied by extraction of copper and zinc in the form of commercial products. Copper extraction by cementation with metallic iron was studied. It was shown that this method could allow extracting 94–95% copper into a concentrate. The role of resolution processes of precipitated copper in cementation was established. To reduce the consumption of iron and to improve the quality of precipitated copper, it is proposed to carry out cementation in a washbox. The copper content in the concentrate was 27–28%, while the specific consumption of iron was 7.0–7.5 kg/kg of copper. During the studies of material composition of copper precipitate, the formation of cuprospinel, goethite, and bassanite phases and the precipitation of basic aluminum sulfates were detected. The extraction of zinc from the solution after copper removal was studied by precipitation with sodium sulfide. As a result, a concentrate was obtained with a zinc content of 49.6–50.9% with extraction of 99%. The process regularities for zinc concentrate dehydration were studied. By optimizing the crystallization conditions of the precipitate, a minimum final cake moisture content of 40–42% was reached. A process flow diagram for acidic underspoil water, including copper cementation and the precipitation of zinc sulfide, was developed. According to this diagram, the mother solution from zinc precipitation should be treated with lime until the pH reaches 10–10.5, followed by slurry settling. As a result, clean water of the following composition is obtained, mg/liter: 0.05 zinc, 0.01 copper, 0.02 aluminum, < 0.02 iron, 0.05 manganese. These values correspond to the quality of drinking water according to GOST R 51232-98.





Similar content being viewed by others
References
M. G. Viduetskii, I. F. Garifulin, V. A. Mal’tsev, and A. P. Purgin, “A technology of cleaning mine, underspoil, reused, and sewage water of mining and smelting facilities,” Tsvetn. Metally, No. 8, 9–14 (2017).
A. M. Pan’shin, M. G. Viduetskii, A. P. Purgin, et al., “Developing a technology of cleaning mine water use a KFM-series pneumatic flotation cell,” Tsvetn. Metally, No. 10, 93–97 (2014).
V. A. Chanturiya and A. P. Kozlov, “Innovative processes of advanced and comprehensive processing of man-made mineral raw materials,” in: Proc. Conf. “Tekhnogen-2012” on Fundamentals of Technologies of Processing and Recycling Man-Made Waste [in Russian], Ekaterinburg (2012), pp. 20–23.
S. A. Yakornov, A. M. Pan’shin, P. A. Kozlov, and D. A. Ivakin, “Current state of the art in technologies of leaching dusts of the iron and steel industry and the products of their pyrometallurgical processing (acid, ammonium, and alkaline technologies),” Tsvetn. Metally, No. 5, 37–43 (2017).
S. E. Klyain, P. A. Kozlov, and S. S. Naboichenko, Extraction of Zinc from Ore Raw Materials [in Russian], Izd. UGTU-UPI, Ekaterinburg (2009).
M. I. Alkatsev, Cementation Processes in Nonferrous Metallurgy [in Russian], Metallurgiya, Moscow (1981).
E-S. Z. El-Ashtoukhy and M. H. Abdel-Azi, “Removal of copper from aqueous solutions by cementation in a bubble column reactor fitted with horizontal screens,” Int. J. Mineral Process.,121, 65–69 (2013).
V. Cala-Rivero, J. C. Arranz-Gonzalez, V. Rodriguez-Gomez, et al., “A preliminary study of the formation of efflorescent sulfate salts in abandoned mining areas with a view to their harvesting and subsequent recovery of copper,” Minerals Eng.,129, 37–40 (2018).
Sajeda A. Al-Saydeh, Muftah H. El-Naas, and Syed J. Zaidi, “Copper removal from industrial wastewater: A comprehensive review,” J. Industr. Eng. Chemistry,56, 35–44 (2017).
M. Karavasteva, “Kinetics and deposit morphology of copper cementation onto zinc, iron and aluminium,” Hydrometallurgy,76, No. 1–2, 149–152 (2005).
G. M. Vol’dman and A. N. Zelikman, Theory of Hydrometallurgical Processes [in Russian], Moscow (2003).
B. D. Khalezov, Studies and Development of the Technology of Heap Leaching of Copper and Copper-Zinc Ores [in Russian], PhD Thesis, 05.16.02, Ekaterinburg (2008).
A. E. Lewis, “Review of metal sulphide precipitation,” Hydrometallurgy, No. 2, 222–234 (2010).
B. D. Khalezov, N. A. Vatolin, L. A. Ovchinnikova, and G. A. Pavlichenko, “Studying the extraction of copper and zinc sulfides from copper–zinc sulfate solutions,” GIAB, No. 1, 261–265 (2005).
L. P. Wang and Y. J. Chen, “Sequential precipitation of iron, copper and zinc from wastewater for metal recovery,” J. Envir. Eng., No. 1, 1–11 (2019).
L. P. Wang, J. Ponou, S. Matsuo, et al., “Selective precipitation of copper and zinc over iron from acid mine drainage by neutralization and sulfidization for recovery,” Soc. Materials Eng. for Resources for Japan, No. 2, 136–140 (2014).
G. G. Chuyanov, Auxiliary Beneficiation Processes. Dehydration and Dust Collection [in Russian], Izd. UGGU, Ekaterinburg (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 63, No. 11, pp. 8–14, November, 2019.
Rights and permissions
About this article
Cite this article
Klyushnikov, A.M. Study of Copper and Zinc Extraction from Underspoil Water. Metallurgist 63, 1135–1143 (2020). https://doi.org/10.1007/s11015-020-00933-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-020-00933-w