Abstract
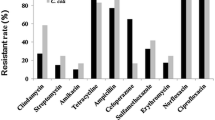
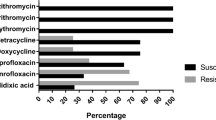
The present study aimed to isolate Arcobacter spp. and Campylobacter spp. from beef meat samples including cattle carcasses, cube meat and minced meat, and to determine the antibacterial susceptibility and genetic diversity of the recovered isolates. One hundred beef carcass surface samples from slaughterhouses and 100 beef meat samples (50 samples of cube meat and 50 minced meat) taken from different retail units were analysed. Of the examined samples, 17 (8.5%) and 43 (21.5%) were positive for Arcobacter spp. and Campylobacter spp. respectively. Twenty Arcobacter and 53 Campylobacter isolates were obtained from positive samples. Both Arcobacter and Campylobacter were concurrently isolated from 7 (3.5%) of the 17 positive samples. Arcobacter butzleri (18 isolates) and Campylobacter jejuni (37 isolates) were the most commonly isolated species. The results of Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction revealed extensive genetic heterogeneity among both Arcobacter and Campylobacter isolates. Seventeen and 30 different genotypes were identified in 18 A. butzleri and 37 C. jejuni isolates, respectively. Each of A. cryaerophilus, C. fetus and C. hyointestinalis isolates had two genotypes. Three and seven genotypes were identified in five C. lari and in seven C. coli isolates, respectively. While 5% of 20 Arcobacter isolates were resistant to amoxicillin-clavulanic acid, 5% of the isolates were resistant to neomycin. Of the 53 Campylobacter isolates, 9.43%, 22.64%, 7.54% and 3.77% were resistant to enrofloxacin, neomycin, tetracycline and streptomycin, respectively. Contaminated beef carcasses, cube meat and minced meat with various species and subspecies of arcobacters and campylobacters may pose a risk factor for human infections. Our study reveals the necessity of improving the hygiene quality in slaughterhouses and other meat processing units as there are different sources of contamination.



Similar content being viewed by others
References
Aarestrup FM, Nielsen EM, Madsen M, Engberg J (1997) Antimicrobial susceptibility patterns of thermophilic Campylobacter spp. from humans, pigs, cattle, and broilers in Denmark. Antimicrob Agents Chemother 41:2244–2250
Abay S, Kayman T, Hizlisoy H, Aydin F (2012) In vitro antibacterial susceptibility of Arcobacter butzleri isolated from different sources. J Vet Med Sci 74:613–616
Abay S, Kayman T, Otlu B, Hizlisoy H, Aydin F, Ertas N (2014) Genetic diversity and antibiotic resistance profiles of Campylobacter jejuni isolates from poultry and humans in Turkey. Int J Food Microbiol 178:29–38
Aksu H, Bostan K, Aydın A (1997) İstanbul’da tüketime sunulan ham kıymalarda Campylobacter jejuni’nin mevcudiyeti üzerine bir araştırma. YYU Vet Fak Derg 8:102–104
Aydin F, Gumuşsoy KS, Atabay HI, Iça T, Abay S (2007a) Prevalence and distribution of Arcobacter species in various sources in Turkey and molecular analysis of isolated strains by ERIC-PCR. J Appl Microbiol 103:27–35
Aydin F, Gumussoy KS, Ica T, Sumerkan B, Esel D, Akan M, Ozdemir A (2007b) The prevalence of Campylobacter jejuni in various sources in Kayseri, Turkey, and molecular analysis of isolated strains by PCR-RFLP. Turk J Vet Anim Sci 31:13–19
Balamurugan S, Nattress FM, Baker LP, Dilts BD (2011) Survival of Campylobacter jejuni on beef and pork under vacuum packaged and retail storage conditions: examination of the role of natural meat microflora on C. jejuni survival. Food Microbiol 28:1003–1010
Bauer AW, Kirby WMM, Sherris JC, Turek M (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45:493–496
Bostan K, Aksu H, Özgen O, Uğur M (2001) Effects of refrigerated and frozen storage on the survival of Campylobacter jejuni in ground and cubed beef. Pak J Biol Sci 4:888–890
Bostan K, Aydın A, Ang MK (2009) Prevalence and antibiotic susceptibility of thermophilic Campylobacter species on beef, mutton, and chicken carcasses in Istanbul, Turkey. Microb Drug Resist 15:143–149
Cloak OM, Duffy G, Sheridan JJ, Blair IS, McDowell DA (2001) A survey on the incidence of Campylobacter spp. and the development of a surface adhesion polymerase chain reaction (SA-PCR) assay for the detection of Campylobacter jejuni in retail meat products. Food Microbiol 18:287–298
CLSI (2008) Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard-third edition M31-A3 vol. 28 no. 8 replaces M31-A2 vol. 22 no. 6. (Wayne, PA)
CLSI (2010) Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, twentieth informational supplement M100-S20. Vol. 30, no. 1 replaces M100-S19 vol. 29 no. 3. Wayne, Pennsylvania
Collado L, Figueras MJ (2011) Taxonomy, epidemiology, and clinical relevance of the Genus Arcobacter. Clin Microbiol Rev 24:174–192
Córdoba-Calderón O, Redondo-Solano M, Castro-Arias E, Arias-EchandI ML (2017) Arcobacter isolation from minced beef samples in Costa Rica. J Food Protoc 80:775–778
Diker KS (1985) Studies on the identification of Campylobacter species isolated from sheep and cattle. Doğa Bilim Dergisi 9:232–240
Duffy LL, Fegan N (2012) Prevalence and concentration of Arcobacter spp. on Australian beef carcasses. J Food Protoc 75:1479–1482
Elmalı M, Can HY (2017) Occurence and antimicrobial resistance of Arcobacter species in food and slaughterhouse samples. Food Sci Technol Camp 37(2):280–285
Ferreira S, Queiroz JA, Oleastro M, Domingues FC (2016) Insights in the pathogenesis and resistance of Arcobacter: a review. Crit Rev Microbiol 42:364–383
González M, Mikkelä A, Tuominen P, Ranta J, Hakkinen M, Hänninen ML, Llarena AK (2016) Campylobacter spp. in the food chain and in the environment. Evira’s Res Rep 2:25–30
Grau FH (1988) Campylobacter jejuni and Campylobacter hyointestinalis in the intestinal tract and on the carcasses of calves and cattle. J Food Protoc 51:857–861
Gregory M, Klein B, Sahin O, Girgis G (2018) Isolation and characterization of Campylobacter hepaticus from layer chickens with spotty liver disease in the United States. Avian Dis 62(1):79–85
Hakkinen M, Heiska H, Hänninen ML (2007) Prevalence of Campylobacter spp. in cattle in Finland and antimicrobial susceptibilities of bovine Campylobacter jejuni strains. Appl Environ Microbiol 73:3232–3238
Hong J, Kim JM, Jung WK, Kim SH, Bae W, Koo HC, Gil J, Kim M, Ser J, Park YH (2007) Prevalence and antibiotic resistance of Campylobacter spp. isolated from chicken meat, pork, and beef in Korea, from 2001 to 2006. J Food Protoc 70:860–866
Houf K, Tutenel A, De Zutter L, Van Hoof J, Vandamme P (2000) Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol Lett 193:89–94
Houf K, de Zutter L, van Hoof J, Vandamme P (2002) Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl Environ Microbiol 68:2172–2178
Hsu TT, Lee J (2015) Global distribution and prevalence of Arcobacter in food and water. Zoonoses Public Health 62:579–589
ISO (2006) Horizontal method for detection and enumeration of Campylobacter spp.—part 1: detection method. ISO 10272- 1:2006, CH-1211 Geneve, Switzerland
Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM (2015) Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720
Kabeya H, Maruyama S, Morita Y, Ohsuga T, Ozawa S, Kobayashi Y, Abe M, Katsube Y, Mikami T (2004) Prevalance of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. Int J Food Microbiol 90:303–308
Kaur T, Singh J, Huffman MA, Petrzelková KJ, Taylor NS, Xu S, Dewhirst FE, Paster BJ, Debruyne L, Vandamme P, Fox JG (2011) Campylobacter troglodytis sp. nov., isolated from feces of human-habituated wild chimpanzees (Pan troglodytes schweinfurthii) in Tanzania. Appl Environ Microbiol 77(7):2366–2373
Kayman T, Abay S, Hızlısoy H (2013) Identification of Campylobacter spp. isolates with phenotypic methods and multiplex polymerase chain reaction and their antibiotic susceptibilities. Mikrobiyol Bul 47:230–239
Kramer JM, Frost JA, Bolton FJ, Wareing DRA (2000) Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J Food Protoc 63:1654–1659
Lane DJ (1991) 16S/23S rRNA Sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lee MH, Cheon DS, Choi S, Lee BH, Jung JY, Choi C (2010) Prevalence of Arcobacter species isolated from retail meats in Korea. J Food Protoc 73:1313–1316
Little CL, Richardson JF, Owen RJ, de Pinna E, Threlfall EJ (2008) Campylobacter and Salmonella in raw red meats in the United Kingdom: prevalence, characterization and antimicrobial resistance pattern, 2003–2005. Food Microbiol 25:538–543
Liu F, Ma R, Wang Y, Zhang L (2018) The clinical importance of Campylobacter concisus and other human hosted Campylobacter species. Front Cell Infect Microbiol 8:1–22
Madden RH, Espie WE, Moran L, McBride J, Scates P (2001) Occurrence of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella and Campylobacter spp. on beef carcasses in Northern Ireland. Meat Sci 58:343–346
Milesi S (2010) Università degli Studi di Milano graduate school of veterinary sciences for animal health and food safety Doctoral Program in Animal Nutrition and Food Safety Academic Year: 2009–2010, pp 50–51
Modirrousta S, Shapouri R, Rezasoltani S, Molaabaszadeh H (2016) Prevalence of Campylobacter spp. and their common serotypes in 330 cases of red-meat, chicken-meat and egg-shell in Zanjan City, Iran. Infect Epidemiol Med 2:8–10
Nieva-Echevarria B, Martinez-Malaxetxebarria I, Girbau C, Alonso R, Fernández-Astorga A (2013) Prevalence and genetic diversity of Arcobacter in food products in the North of Spain. J Food Protoc 76:1447–1450
Ongor H, Cetinkaya B, Acık MN, Atabay HI (2004) Investigation of arcobacters in meat and faecal samples of clinically healthy cattle in Turkey. Lett Appl Microbiol 38:339–344
Pérez-Cataluña A, Salas-Massó N, Diéguez AL, Balboa S, Lema A, Romalde JL, Figueras MJ (2018) Revisiting the taxonomy of the Genus Arcobacter: getting order from the Chaos. Front Microbiol 9:2077
Rahimi E, Ameri M, Kazemeini HR (2010) Prevalence and antimicrobial resistance of Campylobacter species isolated from raw camel, beef, lamb and goat meat in Iran. Foodborne Pathog Dis 7:443–447
Rahimi E, Ameri M, Alimoradi M, Chakeri A, Bahrami AR (2013) Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from raw camel, beef, and water buffalo meat in Iran. Comp Clin Pathol 22:467–473
Ramees TP, Dhama K, Karthik K, Rathore RS, Kumar A, Saminathan M, Tiwari R, Malik YS, Singh RK (2017) Arcobacter: an emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control-a comprehensive review. Vet Q 37:136–161
Ramonaitė S, Rokaitytė A, Tamulevičienė E, Malakauskas A, Alter T, Malakauskas M (2013) Prevalence, quantitative load and genetic diversity of Campylobacter spp. in dairy cattle herds in Lithuania. Acta Vet Scand 5:55–87
Sammarco ML, Ripabelli G, Fanelli I, Grasso GM, Tamburro M (2010) Prevalence and biomolecular characterization of Campylobacter spp. isolated from retail meat. J Food Protoc 73:720–728
Savasan S, Ciftci A, Diker S (2004) Emergence of quinolone resistance among chicken isolates of Campylobacter in Turkey. Turk J Vet Anim Sci 28:391–397
Scullion R, Harrington CS, Madden RH (2006) Prevalence of Arcobacter spp. in raw milk and retail raw meats in northern Ireland. J Food Protoc 69:1986–1990
Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P (2011) Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol 27:1–12
Villarruel-López A, Márquez-González M, Garay-Martínez LE, Zepeda H, Castillo A, Mota de la Garza L, Murano EA, Torres-Vitela R (2003) Isolation of Arcobacter spp. from retail meats and cytotoxic effects of isolates against vero cells. J Food Protoc 66:1374–1378
Vipham J, Brooks C, Miller M, Brashears M, Echeverry AA (2016) Survey of Campylobacter in beef cuts at retail. Beef research online. http://www.beefresearch.org/CMDocs/BeefResearch/Safety_Project_Summaries/FY09_A_survey_of_Campylobacter_in_beef_cuts.pdf. Accessed 12 Dec 2016
Wang G, Clark CG, Taylor TM, Pucknell C, Barton C, Price L, Woodward DL, Rodgers FG (2002) Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J Clin Microbiol 40:4744–4747
Zacharow I, Bystron J, Wałecka-Zacharska E, Podkowik M, Bania J (2015) Prevalence and antimicrobial resistance of Arcobacter butzleri and Arcobacter cryaerophilus isolates from retail meat in Lower Silesia region, Poland. Pol J Vet Sci 18:63–69
Funding
Data relating to the isolation, identification and antibacterial susceptibility of the Campylobacter spp. in this study belongs to Aydın Yağiz’s MSc thesis. Aydın Yağiz’s MSc thesis was supported by the Scientific Research Council of Erciyes University, Kayseri, Turkey (Project no. TSY-11-3415). The authors would like to thank the Scientific Research Council of Erciyes University for the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aydin, F., Yağiz, A., Abay, S. et al. Prevalence of Arcobacter and Campylobacter in beef meat samples and characterization of the recovered isolates. J Consum Prot Food Saf 15, 15–25 (2020). https://doi.org/10.1007/s00003-019-01268-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-019-01268-8