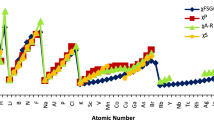
Abstract
Electronegativity (χ) is an important physico-chemical concept to study the chemical structure and reactivity. Although, the conundrum related to measurement of electronegativity still persists. In view of this fact, a simple yet rigorous scale of electronegativity (χ), invoking an inverse relationship with atomic nucleophilicity index (N), has been proposed for 103 elements of the periodic table. The computed data follows periodicity distinctly satisfying all the sine qua non of a standard scale of electronegativity. Further, electronegativity values display a sound similarity with the standard electronegativity scales validating the suitability of the proposed model. Molecular electronegativities of some polyatomic molecules have also been calculated using the proposed scale of electronegativity.
Graphic Abstract









Similar content being viewed by others
References
Accorinti, H.L.: Incompatible models in chemistry: the case of electronegativity. Found. Chem. 21, 71–81 (2019)
Allen, L.C.: Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J. Am. Chem. Soc. 111, 9003–9014 (1989)
Allen, L.C., Knight, E.T.: Electronegativity: why has it been so difficult to define? J. Mol. Struct. Theochem. 261, 313–330 (1992)
Allred, A.L., Rochow, E.G.: A scale of electronegativity based on electrostatic force. J. Inorg. Nuclear Chem. 5, 264–268 (1958)
Boyd, R.J., Markus, G.E.: Electronegativities of the elements from a nonempirical electrostatic model. J. Chem. Phys. 75, 5385–5388 (1981)
Campodónico, P.R., Aizman, A., Contreras, R.: Empirical scale of nucleophilicity for substituted pyridines. Chem. Phys. Lett. 422, 204–209 (2006)
Cárdenas, C., Heidar-Zadeh, F., Ayers, P.W.: Benchmark values of chemical potential and chemical hardness for atoms and atomic ions (including unstable anions) from the energies of isoelectronic series. Phys. Chem. Chem. Phys. 18, 25721–25734 (2016)
Carey, F.A., Sundberg, R.J.: Advanced Organic Chemistry Part A: Structure and Mechanisms. Springer, New York (2007)
Chattaraj, P.K., Maiti, B.: Reactivity dynamics in atom-field interactions: a quantum fluid density functional study. J. Phys. Chem. A 105, 169–183 (2001)
Chatterjee, A.: Special issue on application of density functional theory in chemical reactions. Int. J. Mol. Sci. 3, 234–236 (2002)
Coulson, C.A.: Critical survey of the method of ionic-homopolar resonance. Proc. R. Soc. Lond. Ser. A 207, 63–73 (1951)
Deb, B.M.: The force concept in chemistry. Rev. Mod. Phys. 45, 22–43 (1973)
Domingo, L.R., Ríos-Gutiérrez, M., Pérez, P.: Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21, 748 (2016)
Donnelly, R.A., Parr, R.G.: Elementary properties of an energy functional of the first-order reduced density matrix. J. Chem. Phys. 69, 4431–4439 (1978)
Fukui, K.: Role of frontier orbitals in chemical reactions. Science 218, 747–754 (1982)
Geerlings, P., De Proft, F., Langenaeker, W.: Conceptual density functional theory. Chem. Rev. 103, 1793–1874 (2003)
Ghosh, D.C.: The scales and concept of electronegativity. J. Indian Chem. Soc. 80, 527–533 (2003)
Ghosh, D.C.: A new scale of electronegativity based on absolute radii of atoms. J. Theor. Comput. Chem. 4, 21–33 (2005)
Ghosh, D.C., Chakraborty, T.: Gordy’s electrostatic scale of electronegativity revisited. J. Mol. Struct. Theochem. 906, 87–93 (2009)
Ghosh, D.C., Islam, N.: Whether electronegativity and hardness are manifest two different descriptors of the one and the same fundamental property of atoms: a quest. Int. J. Quantum Chem. 111, 40–51 (2011)
Gordy, W.: A new method of determining electronegativity from other atomic properties. Phys. Rev. 69, 604–607 (1946)
Gyftopoulos, E.P., Hatsopoulos, G.N.: Quantum-thermodynamic definition of electronegativity. Proc. Natl. Acad. Sci. USA 60, 786–793 (1968)
Iczkowski, R.P., Margrave, J.L.: Electronegativity. J. Am. Chem. Soc. 83, 3547–3551 (1961)
Jaramillo, P., Domingo, L.R., Chamorro, E., Pérez, P.: A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. Theochem. 865, 68–72 (2008)
Jaramillo, P., Pérez, P., Contreras, R., Tiznado, W., Fuentealba, P.: Definition of a nucleophilicity scale. J. Phys. Chem. A 110, 8181–8187 (2006)
Kaya, S., Kaya, C.: A new equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 1052, 42–46 (2015)
Lackner, K.S., Zweig, G.: Introduction to the chemistry of fractionally charged atoms: electronegativity. Phys. Rev. D 28, 1671–1691 (1983)
Legon, A.C., Millen, D.J.: Hydrogen bonding as a probe of electron densities: limiting gas-phase nucleophilicities and electrophilicities of B and HX. J. Am. Chem. Soc. 109, 356–358 (1987)
Luo, Y.R., Benson, S.W.: The covalent potential: a simple and useful measure of the valence-state electronegativity for correlating molecular energetic. Acc. Chem. Res. 25, 375–381 (1992)
Mande, C., Deshmukh, P.: A new scale of electronegativity on the basis of calculations of effective nuclear charges from X-ray spectroscopic data. J. Phys. B 10, 2293–2300 (1977)
Mayr, H., Patz, M.: Scales of nucleophilicity and electrophilicity: a system for ordering polar organic and organometallic reactions. Angew. Chem. Int. Ed. Engl. 33, 938–957 (1994)
Minitab 17 Statistical Software [Computer software] State College, PA. Minitab, Inc. www.minitab.com (2010)
Miranda-Quintana, R.A., Martínez González, M., Ayers, P.W.: Electronegativity and redox reaction. Phys. Chem. Chem. Phys. 18, 22235–22243 (2016)
Mulliken, R.S.: A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 2, 782–793 (1934)
Murphy, L.R., Meek, T.L., Allred, A.L., Allen, L.C.: Evaluation and test of Pauling’s electronegativity scale. J. Phys. Chem. A 104, 5867–5871 (2000)
Nalewajski, R.F.: A study of electronegativity equalization. J. Phys. Chem. 89, 2831–2837 (1985)
Ndassa, I.M., Adjieufack, A.I., Mbadcam Ketcha, J., Berski, S., Ríos-Gutiérrez, M., Domingo, L.R.: Understanding the reactivity and regioselectivity of [3 + 2] cycloaddition reactions between substituted nitrile oxides and methyl acrylate. A molecular electron density theory study. Int. J. Quantum Chem. 117, e25451 (2017)
Noorizadeh, S., Shakerzadeh, E.: A new scale of electronegativity based on electrophilicity index. J. Phys. Chem. A 112, 3486–3491 (2008)
Parr, R.G., Bartolotti, L.: On the geometric mean principle for electronegativity equalization. J. Am. Chem. Soc. 104, 3801–3803 (1982)
Parr, R.G., Donnelly, R.A., Levy, M., Palke, W.E.: Electronegativity: the density functional viewpoint. J. Chem. Phys. 68, 3801–3807 (1978)
Parr, R.G., Szentpály, L., Liu, S.J.: Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999)
Parr, R.G., Yang, W.: Density Functional Theory of Atoms and Molecules. Oxford University Press and Clarendon Press, New York (1989)
Parr, R.G., Yang, W.: Density-functional theory of the electronic structure of molecules. Annu. Rev. Phys. Chem. 46, 701–728 (1995)
Pauling, L.: The nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 54, 3570–3582 (1932)
Pauling, L.: The Nature of the Chemical Bond. Cornell University Press, New York (1960)
Pearson, R.G.: Failure of Pauling’s bond energy equation. Chem. Commun. (London) 2, 65–67 (1968)
Pearson, R.G.: Absolute electronegativity and hardness: application to inorganic chemistry. Inorg. Chem. 27, 734–740 (1988)
Pérez, P., Domingo, L.R., Duque-Noreña, M., Chamorro, E.: A condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J. Mol. Struct. Theochem. 895, 86–91 (2009)
Politzer, P., Grice, M.E., Murray, J.S.: Electronegativities, electrostatic potentials and covalent radii. J. Mol. Struct. Theochem. 549, 69–76 (2001)
Politzer, P., Murray, J.S.: Electronegativity: a perspective. J. Mol. Model. 24, 214 (2018a)
Politzer, P., Murray, J.S.: The Hellmann-Feynman theorem: a perspective. J. Mol. Model. 24, 266 (2018b)
Politzer, P., Weinstein, H.: Some relations between electronic distribution and electronegativity. J. Chem. Phys. 71, 4218–4220 (1979)
Pritchard, H.O., Skinner, H.A.: The concept of electronegativity. Chem. Rev. 55, 745–786 (1955)
Qteish, A.: Electronegativity scales and electronegativity-bond ionicity relations: a comparative study. J. Phys. Chem. Solids 124, 186–191 (2019)
Rahm, M., Zeng, T., Hoffmann, R.: Electronegativity seen as the ground-state average valence electron binding energy. J. Am. Chem. Soc. 141, 342–351 (2018)
Ranjan, P., Chakraborty, T.: Density functional approach: to study copper sulfide nanoalloy clusters. Acta Chim. Slov. 66, 173–181 (2019)
Ruthenberg, K., González, J.C.M.: Electronegativity and its multiple faces: persistence and measurement. Found. Chem. 19, 61–75 (2017)
Sanderson, R.T.: An interpretation of bond lengths and a classification of bonds. Science 114, 670–672 (1951)
Sanderson, R.T.: Partial charges on atoms in organic compounds. Science 121, 207–208 (1955)
Slater, J.C.: Quantum Theory of Molecules and Solids. McGraw-Hill, New York (1963)
Tandon, H., Chakraborty, T., Suhag, V.: A new model of atomic nucleophilicity index and its application in the field of QSAR. Internat J Quant Struct Prop Relat. 4, 99–117 (2019a)
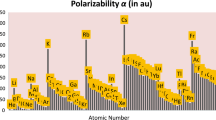
Tandon, H., Chakraborty, T., Suhag, V.: A new scale of atomic static dipole polarizability invoking other periodic descriptors. J. Math. Chem. 57, 2142–2153 (2019b)
Tandon, H., Chakraborty, T., Suhag, V.: A new scale of the electrophilicity index invoking the force concept and its application in computing the internuclear bond distance. J. Struct. Chem. 60, 1725–1734 (2019c)
Acknowledgements
The authors are thankful to Presidency University, Bengaluru; Manipal University Jaipur, Jaipur and BML Munjal University, Gurugram for providing computational and research facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tandon, H., Chakraborty, T. & Suhag, V. A scale of atomic electronegativity in terms of atomic nucleophilicity index. Found Chem 22, 335–346 (2020). https://doi.org/10.1007/s10698-020-09358-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-020-09358-4