The Oldest Representative of the Rove Beetle Tribe Pinophilini (Coleoptera: Staphylinidae: Paederinae), from Upper Cretaceous Burmese Amber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen, Deposition, Photography and Measurements
2.2. Phylogenetic Analyses
2.3. Morphological Characters
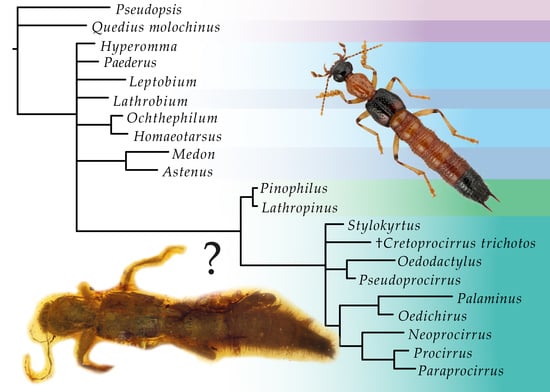
3. Results
3.1. Phylogenetic Analyses
3.2. Systematic Palaeontology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newton, A.F. 2019 StaphBase: Staphylinoidea, Hydrophiloidea, Syntelidae. In world catalog database (version Nov 2018). In Species 2000 & ITIS Catalogue of Life; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., et al., Eds.; accessed on 26th February 2019; Digital resource at www.catalogueoflife.org/col Species 2000; Naturalis: Leiden, The Netherlands.
- Schomann, A.; Solodovnikov, A. Phylogenetic placement of the austral rove beetle genus Hyperomma triggers changes in classification of Paederinae (Coleoptera: Staphylinidae). Zool. Scr. 2017, 46, 336–347. [Google Scholar] [CrossRef]
- Żyła, D.; Yamamoto, S.; Jenkins Shaw, J. Total-evidence approach reveals an extinct lineage of Paederinae rove beetles from Cretaceous Burmese amber. Palaeontology 2019, 62, 935–949. [Google Scholar] [CrossRef]
- Bogri, A.; Solodovnikov, A.; Kypke, J.; Żyła, D. Baltic amber members of the extant Micrillus-Scymbalium lineage of the Paederinae rove beetles (Coleoptera, Staphylinidae) and their systematic and ecological significance. Invertebr. Syst. 2020. (In Press) [Google Scholar]
- Zhang, X.; Zhou, H.Z. How old are the rove beetles (Insecta: Coleoptera: Staphylinidae) and their lineages? Seeking an answer with DNA. Zool. Sci. 2013, 30, 490–502. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.D.; Farrell, B.D.; Caterino, M.S.; Farnum, C.W.; Hawks, D.C.; Maddison, D.R.; Seago, A.E.; Short, A.E.; Newton, A.F.; Thayer, M.K. Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: Forest litter as a stepping stone for diversification of nonphytophagous beetles. Syst. Entomol. 2015, 40, 35–60. [Google Scholar] [CrossRef]
- Herman, L. Generic revision of the Procirrina (Coleoptera: Staphylinidae: Paederinae: Pinophilini). Bull. Am. Museum Natural History 2010, 347, 1–78. [Google Scholar] [CrossRef]
- Faille, A.; Lecoq, J.C. Oedichirus spelaeus n. sp., the first cave dwelling beetle from Madagascar (Coleoptera: Staphylinidae: Paederinae). J. Cave Karst Stud. 2018, 80, 13–18. [Google Scholar] [CrossRef]
- Herman, L. Revision of the New World Species of Oedichirus (Coleoptera: Staphylinidae: Paederinae: Pinophilini: Procirrina). Bull. Am. Museum Natural History 2013, 375, 1–137. [Google Scholar] [CrossRef] [Green Version]
- Assing, V. On the Oedichirus fauna of China (Coleoptera: Staphylinidae: Paederinae). Linzer Biologische Beiträge 2014, 46, 1229–1240. [Google Scholar]
- Rougemont, G. The genus Oedichirus in New Caledonia (Staphylinidae: Paederinae: Pinophilini). Linzer Biologische Beiträge 2018, 50, 537–586. [Google Scholar]
- Chatzimanolis, S. A review of the fossil history of Staphylinoidea. In Biology of Rove Beetles (Staphylinidae)—Life History, Evolution, Ecology and Distribution; Betz, O., Irmler, U., Klimaszewski, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 27–45. [Google Scholar]
- Seevers, C.H. Fossil Staphylinidae in Tertiary Mexican amber (Coleoptera). In Studies of Fossiliferous Amber Arthropods of Chiapas, Mexico; Part II; University of California Publications in Entomology: Oakland, CA, USA, 1971; Volume 63, pp. 77–86. [Google Scholar]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A modular system for evolutionary analysis. Version 3.51. 2018. Available online: http://www.mesquiteproject.org (accessed on 21 October 2019).
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001, 50, 913–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.; Xie, D.; Baele, G.; Suchard, M. Posterior Summarization in Bayesian Phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.L.; Geneva, A.J.; Lanfear, R. RWTY (R We There Yet): An R package for examining convergence of Bayesian phylogenetic analyses. Mol. Biol. Evolut. 2017, 34, 1016–1020. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- Brunke, A.J.; Żyła, D.; Yamamoto, S.; Solodovnikov, A. Baltic amber Staphylinini (Coleoptera: Staphylinidae: Staphylininae): A rove beetle fauna on the eve of our modern climate. Zool. J. Linn. Soc. 2019, 187, 166–197. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.J. Burmese (Myanmar) amber checklist and bibliography 2018. Palaeoentomology 2019, 2, 22–84. [Google Scholar] [CrossRef]
- Newton, A.F.; Thayer, M.K.; Ashe, J.S.; Chandler, D.S. 22. Staphylinidae Latreille, 1802. In American Beetles; Arnett, R.H., Thomas, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2000; Volume 1, pp. 272–418. [Google Scholar]
- Grimaldi, D.A.; Engel, M.S.; Nascimbene, P.C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit. 2002, 3361, 1–72. [Google Scholar] [CrossRef] [Green Version]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkins Shaw, J.; Wang, B.; Bai, M.; Żyła, D. The Oldest Representative of the Rove Beetle Tribe Pinophilini (Coleoptera: Staphylinidae: Paederinae), from Upper Cretaceous Burmese Amber. Insects 2020, 11, 174. https://doi.org/10.3390/insects11030174
Jenkins Shaw J, Wang B, Bai M, Żyła D. The Oldest Representative of the Rove Beetle Tribe Pinophilini (Coleoptera: Staphylinidae: Paederinae), from Upper Cretaceous Burmese Amber. Insects. 2020; 11(3):174. https://doi.org/10.3390/insects11030174
Chicago/Turabian StyleJenkins Shaw, Josh, Bo Wang, Ming Bai, and Dagmara Żyła. 2020. "The Oldest Representative of the Rove Beetle Tribe Pinophilini (Coleoptera: Staphylinidae: Paederinae), from Upper Cretaceous Burmese Amber" Insects 11, no. 3: 174. https://doi.org/10.3390/insects11030174