Effect of UV-Absorbing Nets on the Performance of the Aphid Predator Sphaerophoria rueppellii (Diptera: Syrphidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Aphids and Syrphids
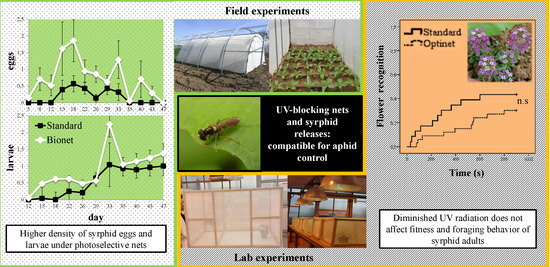
2.2. Field Experiments
2.2.1. Experimental Design
2.2.2. Aphid and Syrphid Samplings
2.3. Effects of UV on Fitness-Related Parameters and Foraging Behavior
2.3.1. Fitness-Related Parameters
2.3.2. Foraging Behavior
2.4. Statistical Analysis
3. Results
3.1. Field Experiments
3.1.1. Light Properties and Environmental Conditions
3.1.2. Light Properties and Environmental Conditions
3.1.3. Density of Syrphid Eggs
3.1.4. Density of Syrphid Larvae
3.2. Effects of UV on Fitness-Related Parameters and Foraging Behavior
3.2.1. Fitness-Related Parameters
3.2.2. Foraging Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramakers, P.M.J. IPM Program for Sweet Pepper. In Biocontrol in Protected Culture; Heinz, K.M., Driesche, V., Parrella, M.P., Eds.; Ball Publishing: Batavia, IL, USA, 2004; pp. 439–455. [Google Scholar]
- Blümel, S. Biological Control of Aphids on Vegetable Crops. In Biocontrol in Protected Culture; Heinz, K.M., Driesche, V., Parella, M.P., Eds.; Ball Publishing: Batavia, IL, USA, 2004; pp. 297–312. [Google Scholar]
- Byers, J.A. Aphids as Crop. Pests; Van Emden, H.F., Harrington, R., Eds.; CABI: London, UK, 2008; Volume 48, pp. 1219–1220. [Google Scholar]
- Rabasse, J.M.; Steenis, M.J. Biological Control of Aphids. In Integrated Pest and Disease Management in Greenhouse Crops; Albajes, R., Lodovica Gullino, M., Lenteren, J.C., Elad, Y., Eds.; Springer: Amsterdam, The Netherlands, 2002; Volume 14, pp. 235–243. [Google Scholar]
- Chyzik, R.; Dobrinin, S.; Antignus, Y. Effect of a UV-deficient environment on the biology and flight activity of Myzus persicae and its hymenopterous parasite Aphidius matricariae. Phytoparasitica 2003, 31, 467–477. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A. A Biological Pest Control Story. IOBC WPRS Bullet. 2006, 29, 19–24. [Google Scholar]
- Ben-Yakir, D.; Hadar, M.D.; Offir, Y.; Chen, M.; Tregerman, M. Protecting crops from pests using OptiNet (R) screens and ChromatiNet (R) shading nets. Acta Hort. 2008, 770, 205–212. [Google Scholar] [CrossRef]
- Díaz, B.; Fereres, A. Ultraviolet-Blocking Materials as a Physical Barrier to Control Insect Pests and Plant Pathogens in Protected Crops. Pest. Tech. 2007, 1, 85–95. [Google Scholar]
- Coombe, P.E. Visual behavior of the greenhouse-whitefly, Trialeurodes vaporariorum. Physiol. Entomol. 1982, 7, 243–251. [Google Scholar] [CrossRef]
- Scherer, C.; Kolb, G. Behavioral experiments on the visual processing of color stimuli in Pieris brassicae L (Lepidoptera). J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1987, 160, 645–656. [Google Scholar] [CrossRef]
- Legarrea, S.; Diaz, B.M.; Plaza, M.; Barrios, L.; Morales, I.; Vinuela, E.; Fereres, A. Diminished UV radiation reduces the spread and population density of Macrosiphum euphorbiae (Thomas) Hemiptera: Aphididae in lettuce crops. Hortic. Sci. 2012, 39, 74–80. [Google Scholar]
- Legarrea, S.; Weintraub, P.; Plaza, M.; Viñuela, E.; Fereres, A. Dispersal of aphids, whiteflies and their natural enemies under photoselective nets. BioControl 2012, 57, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Van Lenteren, J.C.; Noldus, J.J. Whitefly-plant relationships: Behavioural and ecological aspects. In Whiteflies: Their Bionomics, Pest Status and Management; Gerling, D., Ed.; Intercept Ltd: Hants, UK, 1990; pp. 47–49. [Google Scholar]
- Chiel, E.; Messika, Y.; Steinberg, S.; Antignus, Y. The effect of UV-absorbing plastic sheet on the attraction and host location ability of three parasitoids: Aphidius colemani, Diglyphus isaea and Eretmocerus mundus. BioControl 2006, 51, 65–78. [Google Scholar] [CrossRef]
- Prieto-Ruiz, I.; Garzo, E.; Moreno, A.; Dader, B.; Medina, P.; Vinuela, E.; Fereres, A. Supplementary UV radiation on eggplants indirectly deters Bemisia tabaci settlement without altering the predatory orientation of their biological control agents Nesidiocoris tenuis and Sphaerophoria rueppellii. J. Pest. Sci. 2019, 92, 1057–1070. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Ángeles Marcos-García, M. Prey availability and abiotic requirements of immature stages of the aphid predator Sphaerophoria rueppellii. Biol. Control. 2012, 63, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Pineda, A.; Marcos-Garcia, M.A. Seasonal abundance of aphidophagous hoverflies (Diptera: Syrphidae) and their population levels in and outside Mediterranean sweet pepper greenhouses. Ann. Entomol. Soc. Am. 2008, 101, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops; Wiley-Interscience: Chirchester, UK, 2000. [Google Scholar]
- Minks, A.K.; Harrewijn, P. Aphids—Their Biological, Natural Enemies and Control; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989; Volume C. [Google Scholar]
- Shipp, J.L.; Van Houten, Y.M. Influence of Temperature and Vapor Pressure Deficit on Survival of the Predatory Mite Amblyseius cucumeris (Acari: Phytoseiidae). Environ. Entomol. 1997, 26, 106–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Shipp, J.L. Effect of Temperature and Vapor Pressure Deficit on the Flight Activity of Orius insidiosus (Hemiptera: Anthocoridae). Environ. Entomol. 1998, 27, 736–742. [Google Scholar] [CrossRef]
- Laubertie, E.A.; Wratten, S.D.; Hemptinne, J.L. The contribution of potential beneficial insectary plant species to adult hoverfly (Diptera: Syrphidae) fitness. Biol. Control. 2012, 61, 1–6. [Google Scholar] [CrossRef]
- Pineda, A.; Marcos-Garcia, M.A. Use of selected flowering plants in greenhouses to enhance aphidophagous hoverfly populations (Diptera: Syrphidae). Ann. Soc. Entomol. Fr. 2008, 44, 487–492. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-Garcia, M.A. Feeding preferences of the aphidophagous hoverfly Sphaerophoria rueppellii affect the performance of its offspring. Biocontrol 2014, 59, 427–435. [Google Scholar] [CrossRef]
- Meier, U. BBCH-Monograph. Growth Stages of Plants—Entwicklungsstadien von Pflanzen—Estadios de las Plantas—Developpement des Plantes; Meier, U., Ed.; Blackwell Wissenschafts-Verlag: Berlin, Germany; Wien, Austria, 1997. [Google Scholar]
- Ottoni, E.B. EthoLog 2.2: A tool for the transcription and timing of behavior observation sessions. Behav. Res. Methods Instr. Comput. 2000, 32, 446–449. [Google Scholar] [CrossRef] [Green Version]
- Agresti, A.; Natarajan, R. Modeling clustered ordered categorical data: A survey. Int. Stat. Rev. 2001, 69, 345–371. [Google Scholar] [CrossRef]
- McCulloch, C.E.; Searle, S.R. Generalized, Linear, and Mixed Models; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Survival Analysis: Regression Modeling of Time to Event Data; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Dixon, A.F.G. Parthenogenetic reproduction and the rate of increase in aphids. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 269–287. [Google Scholar]
- Almohamad, R.; Verheggen, F.J.; Haubruge, E. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotechnol. Agron. Soc. 2009, 13, 467–481. [Google Scholar]
- Almohamad, R.; Verheggen, F.; Francis, F.; Haubruge, E. Evaluation of hoverfly Episyrphus balteatus De Geer (Diptera: Syrphidae) oviposition behaviour toward aphid-infested plants using a leaf disc system. Comm. Agr. Appl. Biol. Sci. 2006, 71, 403–412. [Google Scholar]
- Belliure, B.; Michaud, J.P. Biology and behavior of Pseudodorus clavatus (Diptera: Syrphidae), an important predator of citrus aphids. Ann. Entomol. Soc. Am. 2001, 94, 91–96. [Google Scholar] [CrossRef]
- Chandler, A.E. Relationship between aphid infestations and oviposition by aphidophagous Syrphidae (Diptera). Ann. Appl. Biol. 1968, 61, 425. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.; Francis, F.; Haubruge, E. Impact of aphid colony size and associated induced plant volatiles on searching and oviposition behaviour of a predatory hoverfly. Belg. J. Entomol. 2008, 10, 17–26. [Google Scholar]
- Budenberg, W.J.; Powell, W. The role of honeydew as an ovipositional stimulant for 2 species of syrphids. Entomol. Exp. Appl. 1992, 64, 57–61. [Google Scholar] [CrossRef]
- Courtney, S.P.; Chen, G.K.; Gardner, A. A general model for individual host selection. Oikos 1989, 55, 55–65. [Google Scholar] [CrossRef]
- Banks, C.J. Effects of Insect Predators on Small Populations of Aphis Fabae in Field. Entomol. Exp. Appl. 1968, 11, 169–176. [Google Scholar] [CrossRef]
- Kan, E. Assessment of Aphid Colonies by Hoverflies. II Pea Aphids and 3-Syrphid Species, Betasyrphus serarius (Wiedemann), Metasyrphus frequens Matsumura and Syrphus Vitripennis (Meigen) (Diptera, Syrphidae). J. Ethol. 1988, 6, 135–142. [Google Scholar] [CrossRef]
- Kan, E. Assessment of Aphid Colonies by Hoverflies. I Maple Aphids and Episyrphus balteatus (De Geer) (Diptera, Syrphidae). J. Ethol. 1988, 6, 39–48. [Google Scholar] [CrossRef]
- Legarrea, S.; Betancourt, M.; Plaza, M.; Fraile, A.; Garcia-Arenal, F.; Fereres, A. Dynamics of nonpersistent aphid-borne viruses in lettuce crops covered with UV-absorbing nets. Virus Res. 2012, 165, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chambers, R.J. Syrphidae; Elsevier: Amsterdam, The Netherlands, 1988; pp. 259–270. [Google Scholar]
- Chandler, A.E.F. Some aspects of host plant selection in aphidophagous Syrphidae. In Ecology of Aphidophagous Insects. Proceedings of a Symposium in Liblice near Prague; Hodek, I., Ed.; Academia: Prague, Czech Republic, 1966; pp. 113–115. [Google Scholar]
- Colley, M.R.; Luna, J.M. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 29, 1054–1059. [Google Scholar] [CrossRef]
- Verheggen, F.J.; Arnaud, L.; Bartram, S.; Gohy, M.; Haubruge, E. Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol. 2008, 34, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameter | Source of Variation | F | df | p |
|---|---|---|---|---|
| Aphid density | Nethouse (1) | 36.572 | 1, 768 | <0.001 |
| Compartment (nethouse) (2) | 3.854 | 6, 768 | 0.001 | |
| Time (3) | 578.189 | 1, 768 | <0.001 | |
| (1) * (3) | 19.483 | 1, 768 | <0.001 | |
| (2) * (3) | 1.315 | 6, 768 | 0.248 | |
| Syrphid egg density | Nethouse (1) | 4.167 | 1, 623 | 0.042 |
| Compartment (nethouse) (2) | 1.414 | 4, 623 | 0.228 | |
| Time (3) | 2.697 | 1, 623 | 0.101 | |
| (1) * (3) | 0.001 | 1, 623 | 0.976 | |
| (2) * (3) | 0.530 | 4, 623 | 0.714 | |
| Syrphid egg presence-absence | Nethouse (1) | 0.000 | 1, 77 | 0.999 |
| Compartment (nethouse) (2) | 0.551 | 5, 77 | 0.737 | |
| Time (3) | 0.000 | 1, 77 | 1.000 | |
| (1) * (3) | 0.000 | 1, 77 | 1.000 | |
| (2) * (3) | 0.168 | 5, 77 | 0.974 | |
| Syrphid larvae density | Nethouse (1) | 5.368 | 1, 525 | 0.021 |
| Compartment (nethouse) (2) | 2.224 | 5, 525 | 0.051 | |
| Time (3) | 50.918 | 1, 525 | <0.001 | |
| (1) * (3) | 1.539 | 1, 525 | 0.215 | |
| (2) * (3) | 0.916 | 5, 525 | 0.470 | |
| Syrphid larvae presence-absence | Nethouse (1) | 4.808 | 1, 63 | 0.032 |
| Compartment (nethouse) (2) | 0.721 | 5, 63 | 0.610 | |
| Time (3) | 82.345 | 1, 63 | <0.001 | |
| (1) * (3) | 1.396 | 1, 63 | 0.242 | |
| (2) * (3) | 1.229 | 5, 63 | 0.306 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorós-Jiménez, R.; Plaza, M.; Montserrat, M.; Marcos-García, M.Á.; Fereres, A. Effect of UV-Absorbing Nets on the Performance of the Aphid Predator Sphaerophoria rueppellii (Diptera: Syrphidae). Insects 2020, 11, 166. https://doi.org/10.3390/insects11030166
Amorós-Jiménez R, Plaza M, Montserrat M, Marcos-García MÁ, Fereres A. Effect of UV-Absorbing Nets on the Performance of the Aphid Predator Sphaerophoria rueppellii (Diptera: Syrphidae). Insects. 2020; 11(3):166. https://doi.org/10.3390/insects11030166
Chicago/Turabian StyleAmorós-Jiménez, Rocco, María Plaza, Marta Montserrat, M. Ángeles Marcos-García, and Alberto Fereres. 2020. "Effect of UV-Absorbing Nets on the Performance of the Aphid Predator Sphaerophoria rueppellii (Diptera: Syrphidae)" Insects 11, no. 3: 166. https://doi.org/10.3390/insects11030166