Abstract
The speciation, toxicity and bioavailability of trace elements in mine drainage environments can be readily predicted using geochemical modelling, and this is frequently the basis for assessing the likely impacts of mine effluents and efficacy of rehabilitation plans. However, such predictions are rarely validated against observed trace element characteristics after mine rehabilitation is complete. In this study of a former Pb–Zn mine in New Zealand, PHREEQC was used to predict dissolved trace element and sediment-bound speciation for the rehabilitated mine site, and the results were compared to the observed water and sediment quality. For Fe, Mn, Al, Cu, Pb, Zn, Cd, Ni, As, and Sb, it was predicted that only Zn2+ and Cd2+ concentrations would exceed recommended guideline values for ecosystem health. PHREEQC indicated that the pH would have to be raised to > 9.5 to reduce these toxicants to a level fit for ecosystem health. Modelling of potential mineral formation indicated that the waters were saturated with respect to a variety of Fe-, Mn- and Al (oxy)hydroxides at and immediately downstream of the mine site, but were not saturated with respect to any trace element-bearing minerals, or sulfide or carbonate phases. This was consistent with X-ray diffraction and scanning electron microscopy (SEM) observations of the sediment. Sequential extraction of the sediment showed strong associations of Zn, Cu, Pb, As and Sb with iron (oxy)hydroxides. Modelling trace element adsorption onto only hydrous ferric oxide surfaces accurately predicted the adsorption of Zn, Cd, Cu, and Ni, but predictions of Pb and As adsorption were less reliable. Additionally, a strong association between Zn and Mn oxyhydroxide was observed in SEM analysis.
Zusammenfassung
Vorkommen, Toxizität und Bioverfügbarkeit von Spurenelementen in Grubenwasserumgebung können mit geochemischer Modellierung gut vorhergesagt werden, was üblicherweise als Basis für die Abschätzung der wahrscheinlichen Auswirkungen von Grubenwässern und der Wirksamkeit von Sanierungsplänen dient. Allerdings werden solche Prognosen kaum mit beobachteten Spurenelementcharakteristiken nach Abschluss der Sanierung validiert. In dieser Studie zu einem Blei-Zinkerzaltbergbau in Neuseeland wurde PHREEQC eingesetzt, um gelöste Spurenelemente und die Sedimentzusammensetzung für den sanierten Bergbau vorherzusagen und die Ergebnisse wurden mit der beobachteten Wasser- und Sedimentqualität verglichen. Für Fe, Mn, Al, Cu, Pb, Zn, Cd, Ni, As, und Sb war prognostiziert, dass lediglich die Zn2+ und die Cd2+ Konzentrationen die empfohlenen Grenzwerte für ein gesundes Ökosystem überschreiten werden. PHREEQC ergab, dass der pH-Wert auf über 9,5 erhöht werden müßte, um diese Schadstoffe auf ein Niveau entsprechend dem gesunden Ökosystem zu reduzieren. Eine Modellierung der potentiellen Mineralisierung zeigt eine Sättigung der Wässer in Hinblick auf Fe-, Mn- und Al(oxy)Hydoxide im Bergbaubereich bzw. im unmittelbaren Abstrombereich. Eine Sättigung der Wässer hinsichtlich spurenelementführender Minerale sowie Sulfid- und Karbonatphasen konnten im Zuge der Modellierung nicht festgestellt werden. Das geht konform mit den röntgendiffraktometrischen und der rasterelektronenmikroskopischen (SEM) Untersuchungen des Sediments. Ein sequentieller Aufschluss des Sediments zeigt eine enge Vergesellschaftung von Zn, Cu, Pb, As und Sb mit Eisen-(oxy)Hydroxiden. Eine Modellierung der Spurenelementadsorption ausschließlich an die Oberfläche von Eisenhydroxiden prognostiziert die Adsorbtion von Zn, Cd, Cu und Ni präzise, währenddessen die Prognose der Pb- und As-Adsorption weniger zuverlässig war. Darüber hinaus wurde mit SEM eine enge Vergesellschaftung zwischen Zn und Mn-Oxyhydroxid mit beobachtet.
Resumen
La especiación, la toxicidad y la biodisponibilidad de los elementos traza en los entornos de drenaje de minas, pueden predecirse utilizando modelos geoquímicos y esa es, con frecuencia, la base para evaluar los probables impactos de los efluentes de la mina y la eficacia de los planes de rehabilitación. Sin embargo, tales predicciones rara vez se validan contra las características observadas de los elementos traza una vez que se completa la rehabilitación de la mina. En este estudio de una antigua mina de Pb–Zn en Nueva Zelanda, PHREEQC se usó para predecir elementos traza disueltos y la especiación de los mismos unidos a los sedimentos para el sitio de la mina rehabilitada; los resultados se compararon con la calidad observada de agua y sedimentos. Para Fe, Mn, Al, Cu, Pb, Zn, Cd, Ni, As y Sb, se predijo que solo las concentraciones de Zn2+ y Cd2+ superarían los valores recomendados para la salud del ecosistema. PHREEQC indicó que el pH tendría que elevarse a más que 9,5 para reducir estos tóxicos a un nivel adecuado para la salud del ecosistema. El modelado de la formación potencial de minerales indicó que las aguas estaban saturadas con respecto a una variedad de hidróxidos de Fe, Mn y Al (oxi) en el sitio de la mina e inmediatamente aguas abajo, pero no estaban saturadas con respecto a ningún mineral que contenga elementos traza o fases de sulfuro o carbonato. Esto fue consistente con las observaciones de difracción de rayos X y microscopía electrónica de barrido (SEM) del sedimento. La extracción secuencial del sedimento mostró fuertes asociaciones de Zn, Cu, Pb, As y Sb con (oxi)hidróxidos de hierro. El modelado de la adsorción de elementos traza solo sobre superficies de óxido férrico hidratado predijo con precisión la adsorción de Zn, Cd, Cu y Ni, pero las predicciones de adsorción de Pb y As fueron menos precisas. Además, en el análisis SEM se observó una fuerte asociación entre los oxihidróxidos de Zn y Mn.
抽象
矿山废水中微量元素的形态、毒性和生物有效性便于通过地球化学模型预测,此类模型是预测矿山排水影响和评价修复计划有效性的基础。然而,即使在矿山修复完成之后,我们也很少用实际观测资料验证之前的预测。在新西兰铅锌矿研究中,利用PHREEQC预测了修复区可溶态及与沉淀结合态的微量元素,将之与实际水样和沉淀的微量金属含量进行对比。预测显示,在Fe、Mn、Al、Cu、Pb、Zn、Cd、Ni、As和Sb元素中,只有Zn2+和Cd2+浓度超过了推荐的健康生态系统的指导值。同时,需要通过提高pH值至9.5才能将有毒物质含量减小到合适的生态系统健康水平。潜在次生矿物模拟结果表明,矿井及紧邻区域下游水样的Fe、Mn和Al氢氧化物饱和,但是含微量元素的硫化物或碳酸盐并不饱和。该结果与与沉淀物的x射线衍射和扫描电镜(SEM)结果一致。沉淀物的顺序提取试验结果表明,Zn、Cu、Pb、As和Sb与铁氢氧化物密切关联。模拟微量元素仅吸附至铁水合氧化物表面可以准确预测Zn, Cd, Cu和Ni的吸附,但Pb和As的吸附预测效果不好。此外,SEM镜下可观察到Zn赋存与锰氢氧化物存在较强关联性.
Similar content being viewed by others
Introduction
Mining can have severe impacts on the natural environment in the vicinity of a mineral resource (Bradshaw and Chadwick 1980; Cooke and Johnson 2002), including the contamination of local streams with acidic mine waters (Lottermoser 2003). The oxidation of iron sulfide minerals such as pyrite, pyrrhotite, marcasite, and mackinawite create acidic conditions in weathering fluids (Lottermoser 2003; Nordstrom and Alpers 1999), which can then facilitate the dissolution of other minerals, releasing trace elements into downstream environments (Ziemkiewicz et al. 1997). The low pH, high sulfate, trace element-rich discharge can be referred to as acid rock drainage (ARD), if occurring naturally, or acid mine drainage (AMD), if associated with mining activities. This distinction is made because iron sulfide oxidation can be accelerated by mining activities when mineral surfaces are exposed to the atmosphere at a faster rate than would occur naturally (Akcil and Koldas 2006; Nordstrom and Alpers 1999). Neutral pH drainage can also occur where there is either low iron sulfide content in the ore or sufficient acid-neutralizing capacity in the host rock (Banks et al. 1997; Blowes et al. 2003; Warrender et al. 2011).
Freshly formed Fe-, Mn- and Al (oxy)hydroxides readily adsorb many trace elements (Johnson and Hallberg 2005; Watzlaf et al. 2004). Under favorable conditions, such (oxy)hydroxides will coagulate and settle into the sediment, together with the associated trace elements, reducing the immediate toxicity of the water to aquatic life, but forming a reservoir of trace elements in the stream sediments. Spatial or temporal changes in pH, redox conditions, and water chemistry influence trace element adsorption and mineral precipitation processes. Reduced pH and redox conditions, for example, can lead to desorption of adsorbed cations or, if significant enough, the dissolution of oxide phases to which these ions had adsorbed. Additionally, pH and redox conditions when the oxide precipitate is forming can affect how effectively trace elements are scavenged from solution (Dale et al. 2015).
The presence and extent of these processes can be theoretically predicted using geochemical models based on thermodynamic equilibria, providing an indication of expected dissolved trace element speciation and adsorption, mineral precipitation under certain conditions and how this may change if conditions are altered. It is an important tool used in mine remediation planning to determine end points for different remediation techniques and to assess the ongoing toxicity and mobility of trace elements after rehabilitation, without having to carry out expensive and time-consuming field trials. However, there are few instances where geochemical model predictions have been validated against observations at a rehabilitated mine site.
The purpose of this research was therefore to compare geochemically modelled trace element speciation, adsorption, and precipitation to that observed at a rehabilitated former Pb–Zn mine. PHREEQC (Parkhurst and Appelo 2013) was used to predict dissolved and sedimentary trace element speciation and trace element attenuation processes, which was then compared to observed trace element associations in the water and sediment of streams draining the mine site. The reliability of this approach for determining the ongoing environmental impacts of a rehabilitated mine site was then assessed.
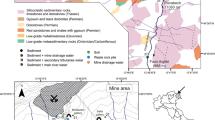
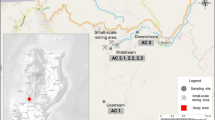
The mine used in this research was the Tui Mine in New Zealand. The Tui mine is a former base metal mine located on the western slopes of Mt. Te Aroha in the Kaimai Ranges. It is 3 km northeast of the township of Te Aroha, at the base of the Coromandel Peninsula (Fig. 1). The mine sits at an altitude of between 300 and 400 m above mean sea level, is surrounded by native bush, and drained by two streams: the Tui Stream on the north side of the mine site and the Tunakohoia Stream on the south side. Both streams are tributaries of the Waihou River. The average rainfall is high, at around 2100 mm/year on Mt. Te Aroha (Harvey and Webster-Brown 2003).
The ore extracted at the Tui Mine came from two mineralized quartz reefs containing sphalerite (ZnS), galena (PbS), pyrite (FeS2), and chalcopyrite (CuFeS2) with minor amounts of cinnabar (HgS), marcasite (FeS2), tetrahedrite [(Cu, Fe)12Sb4S13], and tennantite [(Cu, Fe)12As4S13] (Morrell 1997; Tay 1980; Wodzicki and Weissberg 1970). Although it was first mined for Pb, Au, and Ag in the 1880’s to the early 1900’s, the mine also provided Zn as a flux to gold mining. However, due to primitive ore extraction technology, this early mining did not yield much ore of economic value (Morrell 1997; Tay 1980). In 1967, the mine was reopened by Norpac Mining Ltd to produce Cu, Pb, and Zn (Morrell 1997) but closed again in 1973 when levels of Hg found in the ore made it difficult to find a market (Morrell 1997; Tay 1980). The mine site was not rehabilitated but was left with waste rock dumps, an unstable tailings impoundment at risk of collapse, and contaminants leaching from the mine workings, tailings dam, and tailings impoundment. Load calculations by Harvey and Webster-Brown (2003) identified leachate from the tailings dam as the main source of contamination to Tui Stream, and Adit 5 as the main source of contamination to the Tunakohoia Stream. Consequently, the Tui Mine was recognized as New Zealand’s worst metal-contaminated site (Sabti et al. 2000) with high levels of metal contaminants in streams draining the site (Table 1). Partial site remediation—replacement of the sediment trap downstream of the tailings dam and the construction of a new spillway at the outlet of the sediment trap—was carried out in 2006 after heavy rain caused the former sediment trap to fail (Sharplin 2008). However, the ranking of most contaminated site in the country, along with perceived instability of the tailings dam in a major seismic or weather event, prompted the local regional and district councils to begin full rehabilitation of the site in 2010 (Fairgray et al. 2016). This project was completed in 2013.
The 2010–2013 rehabilitation included plugging the level 5 adit and modifying the level 4 adit to control the amount of mine drainage water discharged (Fairgray et al. 2016). The underground workings were allowed to flood and lime slurry was injected into the workings. The tailings dam was stabilized, mixed with lime, and capped with clean fill to prevent oxygen and water from entering the tailings. Recent monitoring has shown an apparent improvement in water quality (and ecological health) of the streams, although they do still receive some discharge from the adits and the tailings dam (Fairgray et al. 2016). In Tui Stream, for example, the pH is now circumneutral and dissolved Cu and Pb have been reduced to less than 20% of their previous concentrations. However, little is known about how the toxicity of dissolved trace elements has changed, and whether this reduction in concentration is sufficient to allow the recovery of a healthy aquatic ecosystem. There is also concern that trace elements in the sediments may continue to be released from the sediment over time, providing an ongoing source of contamination. This has implications for the rate of recovery of this rehabilitated mine site.
Materials and Methods
Sampling and Analysis
Water and sediment samples were collected at ten sites in the Tui and Tunakohoia streams; four sites on Tui Stream, including a reference site (TuiC) upstream of the mine workings, and five sites on Tunakohoia Stream, and a reference site (TunaC) on the south branch of the Tunakohoia Stream, which is unaffected by mine drainage (Fig. 1). Samples were collected in spring (November 2015) to avoid the extremes of high rainfall and stream flow during winter, and low stream flow in summer.
Measurements of temperature, pH, electrical conductivity (EC), and dissolved oxygen (DO) were made in situ using a HACH HQ40d portable multi parameter meter. Water samples were collected in high density polyethylene (HDPE) centrifuge tubes. One water sample for major ions determination was collected, as well as two water samples for trace element analysis (one unfiltered, for “acid soluble” trace element analysis, and one filtered through a 0.45 µm membrane, for “dissolved” trace element analysis). Both samples were acidified to 1% HNO3 with Aristar-grade concentrated nitric acid prior to analysis. Sediment samples were collected in HDPE bottles, from the top 5 cm of the stream sediment bed.
Water samples were analyzed for major cations and trace elements: Fe, Mn, Al, Cu, Pb, Zn, Cd, Ni, Hg, Sb, and As by inductively coupled plasma mass spectrometry (ICP-MS), with detection limits of 0.02 mg/L for major cations, 1.5 µg/L for Fe and Al, and 0.05 µg/L for all other trace elements except Zn (0.75 µg/L) and Sb (0.20 µg/L). Major anions were determined by high performance ion chromatography, with detection limits of 0.005 mg/L for Cl, 0.020 mg/L for NO3 and 0.200 mg/L for SO4. Dissolved inorganic carbon concentrations were determined by infra-red gas analysis, then reported as HCO3+ as all waters were of a neutral pH (DL = 5.9 mg/L).
Sediment samples were dried at 40 °C and sieved through a 67 µm nylon mesh, then digested in hot nitric acid; the digest was analyzed for trace elements by ICP-MS (as above). Sediment samples were also analyzed by X-ray diffraction (XRD) and scanning electron microscopy (SEM). XRD was performed on a Philips 1130 diffractometer over a 2θ range from 2° to 62°. Clay separates were glycolated by exposure to ethylene glycol vapors for 48 h before being run from 2° to 22° 2θ. SEM was carried out on a JEOL JSM IT-300 variable pressure SEM with an Oxford Aztec SDD energy dispersive X-ray analysis system attached.
Sequential Extraction of Sediments
A sequential chemical extraction scheme based on that of Leleyter and Probst (1999), but including a final hot nitric acid step (Salvarredy-Aranguren et al. 2008), was used to identify the specific solid phases in the sediment that were binding trace elements. The extraction scheme is described in Table 2. A quantity of 0.1 g of sediment (dried and sieved as for total sediment digestion above) was used, with 10 mL of the extractant added to the sediment in most steps (volumes of 3–5 mL were used in steps 5 and 6). The trace element concentrations in fractions 1, 4a, 4b, 4c, 5, and 6 of the sequential extraction were analyzed by ICP-MS, and in fractions 2 and 3 were analyzed by ICP-OES (optical emission spectrometry).
As has been acknowledged in the literature, sequential extractions of this nature can be misleading due to the poor specificity of reagents for target mineral phases and the potential redistribution of elements to other phases during the sequential extraction process (Bacon and Davidson 2008). This has been taken into account when interpreting the results in this study. For example, the sodium acetate extractant (fraction 3) is often referred to as targeting the “carbonate” fraction, but has been renamed here as “low pH leachable” fraction to acknowledge the potential for some trace element cations to desorb from mineral surfaces, as well as carbonates and minor Fe- and Mn-oxyhydroxides to dissolve in this step. The modified nomenclature for target fractions given in Table 2 is used throughout this paper. In addition to this, the sum of each trace element from the fractions in the sequential extraction was compared with the concentration determined by total sediment digest to ensure that redistribution of elements within the fractions was not occurring during the sequential extraction process.
Macroinvertebrate Sampling
Macroinvertebrate samples were collected in December 2014 according to the protocol of Stark et al. (2001). Samples were preserved in the field with 70% ethanol. Samples were then sieved through 500 µm mesh in the laboratory. Invertebrates were counted and identified to the lowest taxonomic level using the identification guides of Winterbourn et al. (2006).
Geochemical Modelling
Dissolved trace element speciation, mineral formation or dissolution, and adsorption reactions was predicted using PHREEQC version 3.3.2 with the WATEQ4F thermodynamic database (Parkhurst and Appelo 2013). PHREEQC is the most commonly used geochemical software for predicting mine effluent chemistry in New Zealand, and is commonly used in effluent chemistry prediction internationally (Rötting et al. 2008; Watten et al. 2005). In this study, PHREEQC was used in the two ways it is most commonly used to predict mine discharge chemistry by mining companies and their consultants;
- 1.
To model current dissolved trace element speciation to determine free ion concentrations (to assess potential toxicity), and to determine speciation change under a regime of changing water quality.
- 2.
To predict dissolved trace element removal through mineral formation and adsorption onto freshly formed hydrous ferric oxide (HFO). For adsorption modelling in a mine remediation context, surface complexation using HFO as the only adsorbing surface is commonly used. This is based on the adsorption data of Dzomback and Morel (1990), which pertains to freshly-formed HFO, with 0.005 strong binding sites/mol Fe, 0.2 weak binding sites/mol Fe, and a specific surface area of 600 m2/g.
PHREEQC was used with the WATEQ4F database for dissolved speciation and adsorption. To model dissolved speciation changes following lime additions (e.g. to the tailings pile and inside Adit 5), 125 µmols of calcite was added stepwise to the stream water from July 2007, as reported by Sharplin (2008) until either the saturation limit (SI = 0.0) of calcite was reached or until CaCO3 was no longer available. 125 µmols was used as it had been determined that this amount would result in the saturation index of calcite being reached but not exceeded.
Batch reactions were run initially to determine which trace element bearing phases were likely to be supersaturated in the solution, and therefore, which minerals were theoretically most likely to precipitate. The minerals that were unlikely to actually form in a low temperature, freshwater environment were eliminated from consideration. This step discounted the formation of minerals such as hematite, cupric ferrite, and cuprous ferrite, which are only formed at high temperatures (Gholinejad et al. 2014; Kolta et al. 1981). The batch reactions were remodeled with the remaining minerals allowed to precipitate when saturated and the constituent dissolved ion concentrations reduced accordingly. The resulting pH change was calculated from the ion balance.
Results
Dissolved Trace Element Concentration, Speciation and Toxicity
Observed Water Quality
Physiochemical water quality at the sampling sites during the November 2015 sampling survey is shown in Table 3. A more complete water chemistry (including all major cations and anions, as needed for speciation modelling) is given in Supplemental Tables A1 and A2 (which accompany the on-line version of this paper).
Remediation at the mine site has effectively raised Tui stream water pH to circumneutral, and lowered conductivity and sulfate concentrations. However, pH was already near neutral in Tunakohoia Stream (Table 1) and the addition of lime in Adit 5 has only had a small effect on pH and little effect on EC or sulfate concentrations when compared with data of Sharplin (2008). Temperature and DO levels are sufficient to support a healthy ecology, with the possible exception of the elevated temperature at Tuna 5, a shallow section of this stream where it passes through open farm land.
Trace element concentrations are shown in Table 4 as both acid-soluble concentrations (in unfiltered, acidified water samples) and as the percentage of dissolved trace element. Acid soluble Hg was consistently below detectable levels, as was Sb at all sites except Tuna 1 (0.25 µg/L) and Tuna 2 (0.13 µg/L). The concentrations of trace elements were considerably higher in Tunakohoia Stream, which still receives drainage from Adit 5, than in Tui Stream.
In New Zealand, any initial assessment of potential trace element toxicity to aquatic life compares acid-soluble concentrations with trigger values in the ANZECC (2000) water quality guidelines. For “moderately disturbed” streams, trigger values represent the highest trace element concentration that will still protect 95% of the aquatic species present. They are referred to as trigger values to indicate they are only the first stage of a toxicity assessment. If they are exceeded, further investigations, such as trace element speciation determination, are required to better assess the toxicity of the contaminant (ANZECC 2000).
Acid-soluble Zn and Cd exceeded ANZECC (2000) trigger values in both streams, but most significantly in Tunakohoia Stream. Notably, the Zn trigger value was even exceeded (slightly) at the reference site (TuiC), upstream of any tailings drainage input, likely reflecting natural elevation due to the weathering of host rock. In addition, the dissolved fraction of these metals constituted 85–100% of the acid-soluble concentration at the contaminated sites, indicating that most of the element was bioavailable.
Of the other trace elements tested, only Cu and Pb exceed trigger values, and then only in the upper reaches of the Tunakohoia Stream. The proportion of dissolved (bioavailable) Cu and Pb is also less than for Cd and Zn, with dissolved Cu constituting 56–89%, and Pb constituting 8–68% of their acid-soluble concentrations. Other trace elements (Fe, Mn, As, and Ni) did not exceed (ANZECC 2000) guidelines; therefore, further assessment of potential toxicity focuses on Zn, Cd, Cu and Pb.
Modelled Dissolved Trace Element Speciation
For dissolved trace element concentrations exceeding ANZECC (2000) trigger values, PHREEQC was used to determine the proportion of the trace element present in the free ion form. The free ion is assumed to be most toxic species to biota according to the free ion activity model (Campbell 1995).
Figure 2 shows PHREEQC predicted dissolved Cd, Cu, Pb, and Zn concentrations. The concentrations of free Cu2+ and Pb2+ ions are predicted to be well below ANZECC (2000) guidelines at all sites, including the upper Tunakohoia Stream, as these metals are complexed by hydroxyl ligands. Free Cu2+ ion constituted only up to 40% of the dissolved Cu in Tunakohoia Stream, and up to 15% in the Tui Stream. Free Pb2+ ion constituted less than 35% of the dissolved Pb in the Tunakohoia Stream and up to 20% in the Tunakohoia Stream.
Speciation of elements exceeding ANZECC (2000) trigger values in Tui and Tunakohoia streams, showing the relevant water quality guideline to protect 95% of aquatic life
However, free Cd2+ and Zn2+ ions remained orders of magnitude higher than the ANZECC (2000) guidelines, for all except the reference sites. The main complexing ligand for Zn and Cd was the sulfate ion, although sulfate complexes made up < 25% of the dissolved Cd and Zn in the Tunakohoia and < 5% in Tui Stream.
Consistency with Ecological Monitoring Data
The modelled concentration of Zn2+ and Cd2+ ions were compared to the results of the ecological surveys carried out at these sites (Fig. 3). In Tunakohoia stream, there appears to be a reduction in ecological taxa at > 3.5 µg/L free Cd ion and > 300 µg/L free Zn ion. However, as high concentrations of Zn and Cd occur simultaneously, it is not possible to state with confidence which of these metals has the greatest detrimental effect on aquatic life. In Tui Stream, ecological effects appear to occur at much lower concentrations, and may be due to other factors, such as the long term low pH that occurred in this stream prior to mine remediation.
Sediment-bound Trace Element Speciation
Observed Sediment Mineralogy and Chemistry
XRD analysis showed that the sediment minerals were predominantly quartz and plagioclase, with chlorite, smectite, illite, and kaolinite clays present as weathering products. No major trace element bearing minerals were identified by XRD. SEM analysis revealed a residual Zn-sulfide particle in Tuna1 sediment just below Adit 5 drainage (Fig. 4), but no other sulfide minerals. No residual carbonates (such as limestone fragments from the remediation activities) or secondary carbonate phases were observed in the sediments. However, strong mineralogical associations between Fe, Pb, and Cu, and between Mn and Zn were observed (examples shown in Fig. 4).
Scanning electron microscope images of; a a sphalerite particle (Zn 35.4% and S 17.2%) from site Tuna1; b a particle showing a strong Mn-Zn association, with high Mn density (c) and high Zn density (d); e a particle showing an Fe–Pb-Cu association, with high Fe density (f), high Pb density (g), and high Cu density (h)
Sediment-bound trace element concentrations are shown in Table 5. ANZECC (2000) interim sediment quality guidelines (ISQG) for aquatic life protection are also shown; ISQG-High is the guideline above which an adverse biological effect is frequently observed, and ISQG-Low is the guideline below which adverse effects rarely occur. For values between these two guidelines, adverse effects occur occasionally (ANZECC 2000; Long et al. 1995). ANZECC (2000) sediment quality guidelines are not available for Fe, Mn, or Al.
Fe concentrations were most elevated in the upper reaches of the streams, closest to the mine discharges, up to 11.4% in Tunakohoia Stream (Tuna 1) and 7.1% in Tui Stream (Tui 1 and 2), but close to background levels elsewhere (3.3–5.2%). Mn was similarly elevated below Adit 5 (3.3%) at Tuna 1 and high immediately below the tailings dam at Tui 1 and 2 (0.34–0.43%), but not significantly elevated downstream. Copious brown Fe oxyhydroxide floc was evident in the upper reaches of both streams; however, the dissolved Fe concentration appeared to increase particularly in Tui Stream. This may, however, be a result of the filtration method as, in the lower reaches, Fe oxide flocs were not as visible, and presumably present as smaller particulates, of which some could have passed through a 0.45 µm filter to register as “dissolved” Fe in the stream.
The Tunakohoia and Tui stream sediments were severely contaminated with Zn, Pb, and Cd, particularly in the upper reaches where concentrations were well above ANZECC (2000) ISQG-High guideline values. Concentrations of up to 4.5% Zn and 1.2% Pb were present in the sediments immediately below Adit 5 drainage (Tuna 1 site) and these sediments also showed high concentrations of Cu, Ni, As, Sb, and Hg. Ni was also high immediately below the tailings dam drainage in Tui Stream and, unexpectedly, at the reference site TunaC.
Modelled Trace Element Precipitation
Modelling carried out using dissolved trace element concentrations showed that once high-temperature phases were discounted, the only trace element-bearing phases that were saturated were oxides of Fe, Mn, and Al. No carbonate minerals of trace elements, such as calcite (CaCO3), rhodochrosite (MnCO3), smithsonite (ZnCO3), otavite (CdCO3), cerrusite (PbCO3), CuCO3, or mixed hydroxycarbonates, such as malachite [Cu2(OH)2CO3] and azurite [Cu3(OH)2(CO3)2] were saturated at the site. Sulfides of the trace elements, such as sphalerite (ZnS), chalcopyrite (CuFeS2), or galena (PbS), were also not saturated in the stream waters due to the redox state of the water favoring SO4 over HS. This indicates that the removal of trace elements from the water column is not likely due to mineral precipitation for Zn, Cd, Cu, or Pb. This also highlights the importance of HFO or ferrihydrite as a key to trace element removal in these streams due to the adsorption of trace elements to the HFO surface.
Sequential Extraction of Sediments
Sequential extraction results confirmed that Fe and Mn were predominantly bound in their respective hydrous oxide phases (Fig. 5a and b). For Mn, this was especially notable at Tuna 1 and Tuna 2 where the total Mn content was very high. A higher proportion of Mn was extracted from the Fe oxide fractions for the Tui stream and Tuna 3 sediment samples. 10–15% of the Fe in the Tui sediments appears to have been in a residual sulfide fraction.
As and Sb were principally extracted from the Fe oxide fractions (Fig. 5g, h), almost to the exclusion of all other fractions. Most of the Zn, Cu, and Pb was extracted in either the low pH leachable, or Fe oxide fractions (Fig. 5c–e). The Fe oxide fraction was of greatest importance downstream in the both streams. Pb and Zn also showed some (less) affinity for the Mn oxide fraction, and Cu and Zn for the “organic” fraction. However, Cu, Zn and Pb in the sulfide and residual fraction was < 3% for all sites, indicating that residual sulfide minerals such as chalcopyrite, sphalerite, and galena make an insignificant contribution to sediment chemistry, despite sphalerite being observed with SEM (Fig. 4a).
In contrast, Cd showed little affiliation with the Fe oxide fractions (Fig. 5f). Cd was instead mainly extracted from the low pH leachable fraction in the upper Tunakohoia Stream sediments, and from the low pH leachable and exchangeable fractions at Tuna 3 and in the Tui Stream sediments. We note that low pH leachable can include leaching from Fe oxide mineral surfaces at pH 4.5.
The sum of trace elements from the fractions extracted was within 30% of the concentration determined by total sediment digest for all samples except for Tuna 2, which had a significantly greater amount of Mn, Cu, Pb, Zn, and Cd for the sequential extraction process. This is more likely due to a non-homogeneous sample than an error in the sequential extraction process.
Modelled Adsorption of Trace Elements onto HFO
PHREEQC was used to characterize the degree of adsorption onto fresh HFO present in the water column and surface sediment. We assumed that the concentration of fresh HFO was equal to the amount of particulate Fe in each water sample, as calculated from the difference between the dissolved and acid soluble Fe concentrations. PHREEQC dissolved trace element concentrations are compared to observed concentrations in Fig. 6.
PHREEQC reliably predicted the dissolved proportion of Zn, Cd, Ni, which were all predominantly dissolved. It also predicted the proportion of dissolved Cu reasonably well (within 20% of observed dissolved concentrations, generally overestimated). PHREEQC overestimated dissolved concentrations to a greater extent at the control sites and the lower catchment waters, indicating additional binding processes (unrelated to HFO adsorption) may be more important for Cu in these waters.
However, PHREEQC was less reliable when predicting the proportion of dissolved Pb and As. For Pb, PHREEQC overestimated the proportion of dissolved Pb for all sites except for the control sites and Tui 3. Although little of the Zn was predicted to adsorb to the HFO, the very high concentration of this trace element in the stream water meant that it still occupied a large portion of the HFO binding sites, inhibiting the adsorption of other trace elements. When Zn adsorption was completely excluded, more Pb bound to the strong binding sites on HFO and the amount of dissolved Pb therefore decreased. The reliability of Pb modelling is therefore likely to be sensitive to competition for binding sites when there are such high concentrations of Zn present.
For As, PHREEQC consistently predicted a high degree of adsorption to the HFO present, which was clearly not occurring as dissolved As was observed to constitute 73–100% of the acid-soluble As. Using only 2–3% of the HFO surface actually available in the model provided a better estimate of the amount of As adsorbed, as was also noted when modelling geothermal As adsorption in lowland rivers (Webster-Brown and Lane 2005).
Modelling Speciation Change with Limestone Addition
The remediation of Tui mine included the addition of crushed limestone to both the tailings pile and Adit 5 to increase pH. This was anticipated to reduce trace element toxicity by complexation, and to facilitate the removal of dissolved trace elements by precipitation and adsorption. If these changes in trace element speciation can be collectively and reliably modelled, this can be used to support remediation decisions.
Where the mineral formed is HFO, the adsorption of cationic trace elements, such as Cu, Pb, Zn, and Cd, is expected to increase with pH, while the adsorption of anionic trace elements such as As decreases under the same conditions. Figure 7 demonstrates this trend for Cu, Pb, Zn, and As adsorption under a regime of increasing pH, for the temperature and HFO concentration present at the Tuna1 site. This shows that where both cationic and anionic trace elements are present at toxic concentrations, modelling can be used to find the stream pH that will result in the optimum cationic and anionic trace elements adsorption onto HFO.
In terms of mineral precipitation, PHREEQC was used to add calcite (CaCO3) stepwise to pre-remediation stream waters, until saturation with respect to calcite was reached. The pre-remediation composition of water at the Tuna1 and Tui1 sites, as reported for July 2007 (Sharplin 2008), was used and the electron potential (pe) was set to 12, to reflect the fast-flowing, fully-oxygenated stream waters. The results are shown in Fig. 8 for pre-remediation Tui 1 stream water, and Fig. 9 for pre-remediation Tuna 1 stream water. The same amount of limestone (125 µmol of CaCO3) added to each stream water yielded quite different results: Tui 1, which had a starting pH of 6.5, could be raised to 9.0 before the solution became saturated with CaCO3, whereas Tuna 1, which had a starting pH of 7.16, could only be raised to 7.67 with the same amount of CaCO3, and did not reach saturation with respect to CaCO3. This shows the diminished return of trying to achieve a pH increase with limestone in a near-neutral, well buffered stream such as Tunakohoia Stream, compared to Tui Stream. The pH actually measured in the streams during this study was around 7.4 for both streams, indicating that the reaction had progressed approximately halfway along these projected trajectory paths.
Modelled changes in dissolved element speciation when a water from Tui 1 (2007 chemistry; Sharplin 2008) is mixed with calcite, simulating the remediation works carried out at the mine site between 2010 and 2013
Modelled changes in dissolved element speciation when a water from Tuna 1 (2007 chemistry; Sharplin 2008) is mixed with calcite, simulating the remediation works carried out at the mine site between 2010 and 2013
In both examples, the predicted concentrations of dissolved Fe were well below the measured concentrations, with the solution being saturated with respect to HFO. This effect became more pronounced at higher pH. The discrepancy between predicted and measured dissolved Fe concentrations in commonly reported and attributed to the ability of fine HFO colloids to pass through 0.45 µm filters and be included in “dissolved” Fe concentrations. Dissolved Fe decreased markedly with increased pH, and was predominantly complexed with hydroxide.
Dissolved Mn and Zn were also predicted to decrease significantly, but only above pH 8 (i.e. in Tui Stream), when water became saturated with respect to rhodocrosite (MnCO3), ZnO, and Zn(OH)2. Free Mn2+ and Zn2+ remained the dominant species. Saturation with respect to rhodochrosite was also achieved in the Tuna1 model, but did not significantly reduce the concentration of Mn or Mn2+. The Mn and Zn concentrations measured in both streams were much less than predicted, indicating that additional precipitation or adsorption reactions were occurring.
For both streams, no change in dissolved Cd, Cu, or Pb concentrations were predicted to occur over the pH range investigated, as no Cd, Cu, or Pb-bearing minerals were predicted to reach saturation. However, the concentrations of Cu2+ and Pb2+ were predicted to decreased in the Tui1 water, as \({\text{Cu}}\left( {\text{OH}} \right)_{2}^{0}\) and \({\text{PbCO}}_{3}^{0}\) became the dominant dissolved species. In the Tuna1 solution, Cu2+ and Pb2+ were already low as \({\text{Cu}}\left( {\text{OH}} \right)_{2}^{0}\) and \({\text{PbCO}}_{3}^{0}\) were the dominant species at the starting pH. Neither the concentration nor percentage of Cd2+ was predicted to decrease significantly in either water. The concentrations of Cd, Cu, and Pb were measured to be much lower at Tui 1, indicating additional precipitation or adsorption reactions occurring. Cd was measured to be much less at Tuna 1, but Cu and Pb concentrations were slightly higher, although similar, to those measured in 2007.
Discussion
The full characterization of the stream water and sediment geochemistry revealed that, even following the rehabilitation of the Tui mine site in 2013, there are still very high concentrations of some trace elements in the water and sediment of Tunakohoia Stream and upper reaches of the Tui stream. In November 2015, dissolved Zn and Cd concentrations were still above guidelines for the protection of aquatic life at all sites except the control site on Tunakohoia Stream, and the dissolved Cu concentration was still above these guidelines in the upper reaches of the stream. The addition of lime slurry to the adits draining to Tunakohoia Stream has not reduced the concentration of either Cd or Zn significantly, and certainly not enough to restore a healthy aquatic ecosystem. Nor has lime addition raised the pH of the adit drainage, which has always been high (circumneutral) relative to the more acidic drainage from the tailings dam (Hendy 1981; Sharplin 2008; Tay 1980; Webster 1995). The rehabilitation of the tailings dam has, however, been more effective, increasing pH to near-neutral and decreasing the concentration of trace elements, such that only Cd and Zn remain above guideline values. Ecological surveys have shown an improvement in Tui Stream health, but overall a strong negative correlation between macroinvertebrate taxa richness and the concentration of Zn2+ and Cd2+ (Fairgray et al. 2016).
Additionally, the concentrations of Cu, Pb, Zn, Cd, Ni, As, Sb, and Hg in the sediment of these streams is elevated and at a level likely impacting aquatic life. In the sediment, strong associations with Mn- or Fe-oxide phases were observed for Zn, Cu, Pb, and Cd, but associations for As and Sb were more exclusively associated with Fe-oxides. The association of Fe, Cu, and Pb was confirmed by SEM elemental mapping while evidence of a Mn–Zn association was also seen. No discrete carbonate minerals were observed by SEM. Additionally, although only a small portion of trace elements in the sediment are currently “exchangeable”, and therefore readily released in a bioavailable form, the sediment is acting as a reservoir for large quantities of trace elements, which could be released should the geochemical conditions (pH or redox) change (Fairgray and Webster-Brown 2017).
It was reasoned that the trace elements extracted in step three of the sequential extraction process included elements that were released at pH 4.5 [and may also include the dissolution of Fe- and Mn- (oxy)hydroxides] but were not strictly associated with carbonate minerals. This is why step three of the sequential extraction was renamed “low pH leachable,” as opposed to “bound to carbonates,” per Leleyter and Probst (1999).
Modelling of Dissolved Trace Element Speciation
PHREEQC was used to assess the toxicity of trace elements to aquatic life by determining the proportion of the element present as the free ion in a similar method to Waters and Webster-Brown (2013). This predicted that most of the dissolved Zn and Cd would be present as free ion and therefore toxic to aquatic life, while the free ion Cu concentration would be below aquatic protection guidelines. This was supported by ecological data showing that taxa richness is reduced at sites with high Zn2+ and Cd2+ concentrations (Fairgray et al. 2016), indicating that PHREEQC could be reliably used to predict the toxicity of Zn and Cd in these environments.
Modelling of Trace Element Speciation in Sediment
PHREEQC has previously been used extensively to understand mine remediation processes and predict efficacy (Bäckström and Sartz 2011; Rötting et al. 2008; Watten et al. 2005). The ability of a geochemical program to predict the precipitation and dissolution behavior of trace elements has been tested by comparing observed sediment characteristics, with predictions based on processes that could control trace element concentrations and mobility in this catchment system. PHREEQC predicted the precipitation of Fe, Mn, and Al oxides, but no trace element bearing minerals under the current water quality conditions. This is consistent with the lack of trace element minerals phases, other than very minor residual primary ZnS (sphalerite) in the sediments. This also supports the conclusion that the Pb, Cu, Zn, and Cd extracted in step three of the sequential extraction were not actually associated with carbonate minerals. PHREEQC also indicated that increasing pH by the addition of more calcite would not significantly change this scenario for Tunakohoia Stream. In Tui Stream, Zn oxide and Mn carbonate precipitation may occur if calcite addition can be used to achieve a high enough pH (> 8.0). Enhanced Fe precipitation could be expected in both catchments. Secondary mineral precipitation is therefore not significantly reducing dissolved trace element concentrations except by adsorption onto the freshly formed secondary Fe, Mn, and Al oxides.
Our results showed that PHREEQC could be used to predict adsorption of trace elements onto HFO by surface complexation, indicating that this was likely an important removal mechanism for Cu, Pb, and As, but less so for Zn, Cd, or Ni. However, removal of Pb (and to a lesser extent Cu) was underestimated and As removal was overestimated; using < 5% of the available HFO better simulated the actual amount of adsorption. This indicates that for Cu and Pb, adsorption to HFO is not the only process binding these metals to the sediment, particularly where HFO concentrations were lower, at the control sites and in the lower reaches of the catchments.
The precipitation of Mn oxides was predicted to be currently occurring in the streams, despite the fact that particulate Mn concentrations (particulate Mn = total acid soluble Mn—dissolved Mn) in the water samples collected were low. However, moderate proportions of Mn, Pb, and Zn were sequentially extracted from the sediment in the Mn oxide fraction. This, combined with the strong association between Mn and Zn observed with SEM, supports the presence of Mn oxide phases, perhaps too fine or only in the sediment where Fe oxides can facilitate their formation (Davies and Morgan 1989). It also indicates that adsorption onto and/or co-precipitation with Mn oxides may be an important metal removal process for Zn and Pb. However, formation of Mn oxides and attenuation of trace elements to Mn oxides is likely to be of less overall importance than formation of and adsorption to Fe (oxy)hydroxides, given their relative abundance.
PHREEQC reliably predicted the formation of secondary Fe and Mn oxide phases and the adsorption of Zn, Cd, and Ni, and to a lesser extent Cu, onto HFO. It under and overestimated the adsorption of Pb and As, respectively. We note that a significant limitation of PHREEQC is that it assumes that the system is at thermodynamic equilibrium. This may not always be the case when fast moving mine drainage streams allow little time for sediment–water interactions to take place. However, if water and sediment chemistry are constant with time, such programs can still provide useful predictions of AMD effects on impacted aquatic environments. In the case of the Tui Mine, for example, modelling of geochemical processes indicates that further mine remediation through calcite addition alone is unlikely to be more effective.
Conclusions
The extent to which mine site restoration has been achieved has been evaluated by geochemical assessments, measurements, and modelling. Mineral precipitate formation is identified as an important metal removal process for Fe and Mn. Adsorption onto these precipitates and the formation of complexes has reduced Cu, Pb, and As concentrations, such that these elements now meet aquatic health guideline values.
Neither the formation of mineral precipitates nor sorption to freshly formed mineral precipitates has reduced Zn and Cd concentrations enough to meet water quality guidelines. Modelling has shown that this is unlikely to be achieved by more of the same rehabilitation methods [i.e. addition of limestone [CaCO3]].
PHREEQC proved to be reliable for predicting Cu, Zn, and Cd concentrations when HFO was determined by the difference between acid soluble and dissolved Fe concentrations, but was less reliable for As and Pb. Modelling also indicated where further characterization of the water and the sediment binding phases was required.
References
Akcil A, Koldas S (2006) Acid mine drainage (AMD): causes, treatment and case studies. J Clean Prod 14:1139–1145
ANZECC (2000) Australian and New Zealand guidelines for freshwater and marine water quality. Australian and New Zealand Environment Conservation Council, Canberra
Bäckström M, Sartz L (2011) Mixing of acid rock drainage with alkaline ash leachates—fate and immobilisation of trace elements. Water Air Soil Pollut 222:377–389
Bacon JR, Davidson CM (2008) Is there a future for sequential chemical extraction? Analyst 133:25–46
Banks D, Younger PL, Arnesen RT, Iversen ER, Banks SB (1997) Mine-water chemistry: the good, the bad and the ugly. Environ Geol 32:157–174
Blowes DW, Ptacek CJ, Jambor JL, Weisener CG (2003) The geochemistry of acid mine drainage. In: Lollar BS, Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 9. Elsevier, New York, pp 149–204
Bradshaw AD, Chadwick MJ (1980) The restoration of land: the ecology and reclamation of derelict and degraded land. University of California Press, Berkeley
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, New York, pp 45–102
Cooke JA, Johnson MS (2002) Ecological restoration of land with particular reference to the mining of metals and industrial minerals: a review of theory and practice. Environ Rev 10:41–71
Dale JG, Stegemeier JP, Kim CS (2015) Aggregation of nanoscale iron oxyhydroxides and corresponding effects on metal uptake, retention, and speciation: I. Ionic-strength and pH. Geochim Cosmochim Acta 148:100–112
Davies SHR, Morgan JJ (1989) Manganese(II) oxidation kinetics on metal oxide surfaces. J Colloid Interface Sci 129:63–77
Dzomback DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York City
Fairgray M, Webster-Brown J (2017) Release of toxic trace elements from contaminated stream sediment at Tui Mine Te Aroha, New Zealand. In: Proceedings of AusIMM New Zealand Branch Annual Conference, Christchurch, New Zealand
Fairgray M, Webster-Brown J, Harding J, Waters AS (2016) Geochemical modelling of metal toxicity in the Tui Mine catchment, Te Aroha, NZ. In: Proceedings of AusIMM New Zealand Branch Annual Conference, Wellington, New Zealand, pp 100–108
Gholinejad M, Karimi B, Mansouri F (2014) Synthesis and characterization of magnetic copper ferrite nanoparticles and their catalytic performance in one-pot odorless carbon-sulfur bond formation reactions. J Mol Catal A Chem 386:20–27
Harvey SA, Webster-Brown JG (2003) Environmentally and publicly acceptable options for remediation at Tui Mine site, Te Aroha. In: Proceedings of AusIMM New Zealand Branch Annual Conference Greymouth, New Zealand
Hendy CH (1981) The Tui Mine—after the miners have left. NZ Environ 29:17–19
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Kolta GA, El-Tawil SZ, Ibrahim AA, Felix NS (1981) Kinetics and mechanism of copper ferrite formation. Thermochim Acta 43:279–287
Leleyter L, Probst J-L (1999) A new sequential extraction procedure for the speciation of particulate trace elements in river sediments. Int J Environ Anal Chem 73:109–128
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Lottermoser BG (2003) Mine wastes. Springer, Germany
Morrell WJ (1997) An assessment of the revegetation potential of base-metal tailings from the Tui Mine, Te Aroha, New Zealand. PhD Thesis, Massey University, New Zealand
Nordstrom DK, Alpers CN (1999) Geochemistry of acid mine waters. In: Plumlee GS, Logsdon MJ (eds) Reviews in economic geology, vol 6. Society of Economic Geologists, Littleton, pp 133–160
Pang L (1995) Contamination of groundwater in the Te Aroha area by heavy metals from an abandoned mine. J Hydrol NZ 33(1):17–34
Parkhurst DL, Appelo CAJ (2013) Description of input and examples for PHREEQC. Version 3-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geological Survey Techniques and Methods, Book 6. USGS, Reston, WV, USA
Rötting TS, Thomas RC, Ayora C, Carrera J (2008) Passive treatment of acid mine drainage with high metal concentrations using dispersed alkaline substrate. J Environ Qual 37:1741–1751
Sabti H, Hossain MM, Brooks RR, Stewart RB (2000) The current environmental impact of base-metal mining at the Tui Mine, Te Aroha, New Zealand. J Roy Soc New Zeal 30:197–207
Salvarredy-Aranguren MM, Probst A, Roulet M, Isaure M-P (2008) Contamination of surface waters by mining wastes in the Milluni Valley (Cordillera Real, Bolivia): mineralogical and hydrological influences. Appl Geochem 23:1299–1324
Sharplin REP (2008) Environmental geochemistry after partial site remediation at Tui Mine, New Zealand. MSc Thesis, University of Auckland, NZ
Stark JD, Boothroyd IK, Harding JS, Maxted JR, Scarsbrook MR (2001) Protocols for sampling macroinvertebrates in wadeable streams. New Zealand Macroinvertebrate Working Group Report No. 1. Ministry for the Environment, Wellington, NZ
Tay KA (1980) Geochemistry and environmental impact of the discharge of heavy metals from the Tui Mine and its tailings. MSc Thesis, University of Waikato, NZ
Warrender R, Pearce NJG, Perkins WT, Florence KM, Brown AR, Sapsford DJ, Bowell RJ, Dey M (2011) field trials of low-cost reactive media for the passive treatment of circum-neutral metal mine drainage in mid-Wales, UK. Mine Water Environ 30:82–89
Waters AS, Webster-Brown JG (2013) Assessing aluminium toxicity in streams affected by acid mine drainage. Water Sci Technol 67:1764–1772
Watten BJ, Sibrell PL, Schwartz MF (2005) Acid neutralization within limestone sand reactors receiving coal mine drainage. Environ Pollut 137:295–304
Watzlaf GR, Schroeder KT, Kleinmann RL, Kairies CL, Nairn RW (2004) The Passive Treatment of Coal Mine Drainage. US Dept of Energy National Energy Technology Laboratory, DOE/NETL-2004/1202
Webster JG (1995) Chemical processes affecting trace metal transport in the Waihou river and estuary, New Zealand. N Z J Mar Fresh 29:539–553
Webster-Brown JG, Lane V (2005) Modeling seasonal arsenic behavior in the Waikato River, New Zealand, vol 915. ACS Symp Series. Oxford University Press, Oxford, pp 253–266
Winterbourn MJ, Gregson KLD, Dolphin CH (2006) Guide to the aquatic insects of New Zealand, 4th edn. Bulletin of the Entomological Society of New Zealand 14
Wodzicki A, Weissberg BG (1970) Structural control of base metal mineralisation at the Tui Mine, Te Aroha, New Zealand. N Z J Geol Geophys 13:610–630
Ziemkiewicz PF, Skousen JG, Brant DL, Sterner PL, Lovett RJ (1997) Acid mine drainage treatment with armored limestone in open limestone channels. J Environ Qual 26:1017–1024
Acknowledgements
The authors thank: the Ministry of Business, Innovation and Employment (MBIE) for funding this research (CRLE 1403 contract); the laboratory staff at Lincoln University (John Revell, Roger Cresswell, and Lynne Clucas) and the University of Canterbury (Rob Stainthorpe) who carried out sample analysis; Mike Flaws (UC) for assistance with SEM imaging; Phil White (Panda Geosciences) for XRD analysis; Prof. Jon Harding, Justin Pomeranz, Dr. Kevin Simon, and Rose Gregersen who accompanied the authors during field work and carried out sampling and identification of macroinvertebrate samples; the Waikato Regional Council for access to the site; and the Iwi Advisory Group for their cultural guidance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fairgray, M.E., Webster-Brown, J.G. & Pope, J. Testing Geochemical Predictions of Trace Element Toxicity and Bioavailability at a Rehabilitated Mine Site. Mine Water Environ 39, 75–92 (2020). https://doi.org/10.1007/s10230-019-00644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-019-00644-y