Abstract
Context
Land-use change and habitat fragmentation are well known drivers of biodiversity declines. In forest birds, it has been proposed that landscape change can cause increased predation pressure that leads to population declines or community change. Predation can also have non-lethal effects on prey, such as creating ‘landscapes of fear’. However, few studies have simultaneously investigated the relative contribution of regional land-use and local management to creating ‘landscapes of fear’.
Objectives
To quantify the relative contribution of regional land-use and local management to the ‘landscape of fear’ in agricultural landscapes.
Methods
Bioacoustic recorders were used to quantify Eurasian Wren Troglodytes troglodytes alarm call rates in 32 naturally replicated broadleaf woodlands located in heterogeneous agricultural landscapes.
Results
Alarm call rates (the probability of an alarm per 10 min of audio) were positively correlated with the amount of agricultural land (arable or pasture) within 500 m of a woodland (effect size of 1) and were higher when livestock were present inside a woodland (effect size of 0.78). The amount of woodland and urban land cover in the landscape also had positive but weak effects on alarm call rates. Woodlands with gamebird management had fewer alarm calls (effect size of − 0.79).
Conclusions
We found that measures of both regional land-use and local management contributed to the ‘landscape of fear’ in agricultural landscapes. To reduce the impact of anthropogenic activities on ‘fear’ levels (an otherwise natural ecological process), land-managers should consider limiting livestock presence in woodlands and creating traditional ‘buffer strips’ (small areas of non-farmed land) at the interface between woodland edges and agricultural fields.
Similar content being viewed by others
Introduction
Habitat loss, fragmentation and land-use change disrupt ecological functioning and cause biodiversity loss (Haddad et al. 2015). While the general impacts are well documented, the mechanistic links between pattern and process can be complex and obscure. Behavioural responses to changes in habitat configuration as a result of habitat fragmentation is one such process that underlies many species’ responses to landscape change. For example, reduced structural connectivity between isolated patches can inhibit individual movement through the landscape and impair dispersal and colonisation behaviour (Haddad et al. 2015). Land-use change can also affect other behaviour, such as predator–prey interactions (Dolman et al. 2007; Thompson 2007). Most work on the relationships between landscape fragmentation, land-use change and predation has focused on lethal effects on prey (e.g. nest predation) (Lahti 2001; Batáry and Báldi 2004) or predator responses (Chalfoun et al. 2002). However, predators can also have non-lethal effects on prey, potentially resulting in substantial fitness costs across an individual’s lifetime (Cresswell 2008). Despite this, the non-lethal effects of predation, such as levels of fear (perceived predation risk), have received relatively limited attention in the context of habitat fragmentation and land-use change.
The non-lethal effects of predators on prey include behavioural trade-offs that can incur fitness costs (Cresswell 2008). For example, prey can experience opportunity costs when they avoid foraging in areas with high predation risk (Hilton et al. 1999), or when time is allocated to predator detection or avoidance at a cost to other activities such as territorial defence or feeding (Krebs 1980; Dunn et al. 2010). Perceived predation risk, where an individual perceives a threat from a predator (e.g. a bird alarm calling when it sees a predator at a distance) can also affect physiology and demography. In Great Tits Parus major, body mass (fat reserves) declined under increased levels of perceived predation risk, even when no actual predation attempts were made (Gosler et al. 1995; Gentle and Gosler 2001). In wild Song Sparrows Melospiza melodia the number of offspring declined by 40% per year solely due to higher perceived predation risk (Zanette et al. 2011), and the number of young reaching independence can decline by more than half (Dudeck et al. 2018). Thus, when individuals perceive high levels of predation risk they can incur multiple physiological, demographic and behavioural costs that could have wider consequences for populations.
Songbird reproductive fitness is strongly mediated by nest predation (see reviews by Lahti 2001; Thompson et al. 2002; Stephens et al. 2003). Predation pressure operates across a hierarchy of spatial scales ranging from the geographic distribution of predators and predator responses to large-scale habitat patterns (i.e. regional land-use), down to local, patch-scale effects on predation risk (e.g. vegetation structure, patch size, edge effects, predator control, livestock presence). In forest passerines, nest predation rates typically increase as forest cover in the landscape declines, probably because densities of predators (e.g. corvids) are higher in agricultural or urban environments that replace forest (Chalfoun 2002; Thompson 2007; Cox et al. 2012). At the local scale, predation rates can increase when nests are closer to patch edges or when patches are smaller and have high edge to area ratios (Lahti 2001; Batáry and Báldi 2004). Very fine scale measures of vegetation structure in the immediate vicinity of the nest site, such as understory cover, can also influence predation rates by affecting nest detectability and nestling provisioning (Dunn et al. 2010; Bellamy et al. 2018). Vegetation structure can also be altered by the presence of livestock (Martin and Possingham 2005; Mandema et al. 2013; Beja et al. 2014). However, predator and prey responses to landscape change are often context-dependent (Chalfoun et al. 2002), and most studies are from North America with few comparative studies in Europe, leading to a call for more research (Dolman et al. 2007; Thompson 2007).
Vocal communication (singing, alarm calling) is a fundamental behaviour used by passerines for territorial defence, advertising individual quality, attracting a mate, signalling predator presence and signalling hunger. Alarm calls often have several functions and the messages they convey can vary throughout the year. Nestling begging behaviour, which is noisy and can attract predators is suppressed by parental alarm calls in the White-browed Scrubwren Sericornis frontalis (Platzen and Magrath 2004). Thus, parental alarm calls can serve as an early warning of predation risk to nestlings. Alarm calls can also convey more subtle messages. In Black-capped Chickadees Poecile atricapilla, experimental presentation of predators showed that there was a correlation between acoustic features of alarm calls and predator body size, and this information was decoded by conspecifics during mobbing behaviour (Templeton et al. 2005). A recent experimental study showed that when Great Tits perceived an increase in predation risk they traded off territorial communication (i.e. male song) with an increase in alarm-call behaviour (Abbey-Lee et al. 2015).
Given the almost ubiquitous importance of vocal behaviour in passerine birds, such negative responses to higher predation risk are likely to be widespread. The factors that drive behavioural responses to perceived predation risk are also likely to be both direct (e.g. increased predator abundance) and indirect, for example through land-use change and its effects on predator behaviour. However, despite considerable research across multiple spatial scales, the relative importance of factors such as edge effects vs regional land-use is rarely quantified. From a land-management perspective, it is important to understand which scales have the largest impact on perceived predation risk, alongside other measures of ecosystem ‘quality’, so that limited resources for conservation can be targeted towards the most effective solutions (e.g. managing woodland structure vs large-scale landscape restoration).
Quantifying perceived predation risk under natural conditions is challenging, firstly because it can be difficult to observe predator–prey interactions and secondly because observer presence can interfere with both predator and prey behaviour. To overcome these challenges, studies of birds typically simulate predation risk (e.g. at feeder stations) by using dummy predators, broadcasting predator vocalizations or using artificial nests (e.g. Gentle and Gosler 2001; Storch et al. 2005; Zanette et al. 2011; Mandema et al. 2013; Beja et al. 2014; Abbey-Lee et al. 2015). Technological advances such as camera traps have also made it possible to detect attempted or actual predation events under natural conditions, for example at songbird nests (Bellamy et al. 2018), which can complement findings from experimental work. In addition, knowledge of bird communication behaviour has been greatly advanced by affordable bioacoustic technology, and automated detectors can now be deployed to record bird song and other communication behaviour at landscape-scales and for long time periods (Blumstein et al. 2011).
Here, we used a natural experiment approach and a model species (Eurasian Wren Troglodytes troglodytes) to assess the effects of regional land-use and local management on perceived predation risk in agricultural landscapes. Specifically, we addressed the following questions: (i) is perceived predation risk correlated with landscape-scale measures of land-use?; (ii) do patch-level woodland management practices (e.g. gamebird management) correlate with perceived predation risk?; and (iii) what is the relative importance of regional land-use vs local management for perceived predation risk?
Methods
Study sites
Thirty-five post-agricultural broadleaf woodlands (Figure S1) were selected from a larger sample of 107 woodlands used by the Woodland Creation and Ecological Networks (WrEN) natural experiment in the UK (Watts et al. 2016). Woodlands patches were identified from the National Forest Inventory digital woodland map (Forestry Commission 2013). The 35 woodlands were later reduced to 32 because no Eurasian Wren alarm calls or songs were detected in one site (suggesting it was unoccupied) and two other woodlands were removed due to missing data.
Patch size is an important predictor of nest predation in woodland birds (Dolman 2012) but our primary aim was to disentangle the relative effects of regional land-use and woodland management on perceived predation risk within a focal patch. We therefore attempted to control for patch size by selecting woodlands of similar size (0.5–2.6 ha), which were ‘naturally replicated’ across landscapes that varied in the amount of agricultural land within a 3 km Geographic Information System (GIS) buffer (measured from the woodland edge). Figure S2 shows frequency plots of land-cover types surrounding the study woodlands. These indicate that, in our study area, agricultural land replaces wooded and semi-natural land-cover. Woodland patches were spaced c.3 km apart, first to ensure that they were spatially independent at the landscape-scale (Eurasian Wren breeding territories are usually < 5 ha; Wesołowski 1983) and secondly because this scale was considered large enough to be correlated with variation in predator abundance, for example.
Study species
The Eurasian Wren is a ubiquitous woodland bird in Great Britain (Balmer et al. 2013) and was recorded in 96% of 101 lowland broadleaf secondary woodlands surveyed in central Scotland and central England in 2015 (Whytock et al. 2017). Nests are dome shaped and located on or near the ground (usually < 5 m) in thick vegetation or cavities, and constructed from dry leaves, moss, grass and other plant material (Wesołowski 1983; Ferguson-Lees et al. 2009). Nest predation is one of the most important factors affecting Eurasian Wren fecundity (Garson 1980; Wesołowski 1983) and a loud and characteristic ‘chattering’ alarm call is made when the nest site is threatened (Ferguson-Lees et al. 2009). Predation events are rarely observed directly and little information is available on Eurasian Wren predators, but the potential predator community in our study areas comprises Sparrowhawk Accipiter nisus, Buzzard Buteo buteo, Tawny Owl Strix aluco, Eurasian Magpie Pica pica, Eurasian Jay Garrullus glandarius, Carrion Crow Corvus corone, Jackdaw Corvus monedula, European weasel Mustela nivalis, stoat Mustela ermine, European badger Meles meles, red fox Vulpes vulpes, grey squirrel Sciurus carolinensis, brown rat Rattus norvegicus, domestic cat Felix sylvestris catus (Baker et al. 2008) and various small Rodentia. Eurasian Wrens also habitually make alarm calls in response to the presence of humans, domestic dogs Canis lupus familiaris and livestock.
Quantifying perceived predation risk
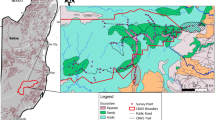
We used Solo audio recorders (Whytock and Christie 2017) to detect Eurasian Wren ‘chattering’ alarm calls (Figure S3) inside the focal woodlands. One Solo was deployed in the centre of each woodland patch (Fig. 1) and set to record audio continuously (24 h 7 days) from 1 to 30 April 2016, when Eurasian Wren territories are established and egg laying occurs (Ferguson-Lees et al. 2009). This method allowed alarm calls (an index of perceived predation risk, see Data analysis) to be detected at high temporal resolutions in focal patches spread across a large geographic area during the core breeding season. We did not explicitly test the distances at which Solo recorders could detect alarm calls, but previous work showed that Willow Warbler Phylloscopus trochilus song could not be detected by Solo recorders beyond approximately 50 m (see electronic supplementary material in Whytock et al. 2017). Since Eurasian Wren ‘chattering’ alarm calls are louder than Willow Warbler song, maximum detection distances were probably < 100 m from the microphone, which is within the scale of our small patches (Fig. 1). The woodlands used in the study are also relatively isolated by design (mean 167.31 m from nearest neighbouring woodland, n = 32 woods) and it was unlikely that we detected alarms beyond the vicinity of the focal patch.
Illustration showing audio recorder placement and approximate alarm call detection distances in three example focal woodlands. Importantly, the detection radius encompasses the focal woodland and woodlands are sufficiently isolated to limit possible detection of calls originating from neighbouring woodland patches
Data analysis
Quantifying alarm call rates
We used an unbiased re-sampling approach to quantify alarm call rates in each woodland. Audio sequences were examined on four equally spaced days in April (9th, 16th, 23rd and 30th) during the core breeding season (Ferguson-Lees et al. 2009). For each site, four 10 min sections of audio (i.e. 40 min) were randomly sampled from three sampling periods per day: (i) the 3 h period after sunrise (morning), (ii) between 1200 and 1400 (midday), and (iii) the 2 h period before sunset (evening). A total of 120 min was therefore sampled per day, totalling 480 min of audio per woodland. We visually annotated and counted the number of alarm calls (Figure S3) in each 10 min section of audio using digital audio spectrograms.
Modeling approach
Counts of alarm calls were low and fewer than 4% (n = 75) of the 10 min audio sections (n = 1680) had more than one alarm call. The lack of variation in the non-zero component of the data prevented us using models that deal with zero inflation (e.g. Brooks et al. 2017). We therefore created a binary response variable of alarm call presence/absence within a 10 min audio section and used logistic generalised linear mixed effects models (GLMMs) to quantify the effects of local and landscape-scale metrics on alarm call presence/absence per 10 min audio section.
All variables used in the regression analysis, summary statistics and their predicted effect (with a short rationale) are given in Table 1. The three landscape variables (proportion agricultural land [arable or pasture], proportion urban land cover and proportion of any woodland land cover; Table 1) were measured using remote sensing data (Morton et al. 2011) at eight spatial scales (20 m, 250 m, 500 m, 1000 m, 1500 m, 2000 m, 2500 m and 3000 m). We found that, within each land cover type, there was high correlation between scales from 20 m to 500 m, and from 1000 m to 3000 m. We therefore chose 500 m and 3000 m as separate ‘near’ and ‘far’ indicators for each of the three landscape variables (Table 1). These were included in the model as continuous fixed effects without interactions to avoid over-fitting.
Five local-scale variables comprising vegetation structure in the patch, management practices inside the patch boundaries and patch shape were used in the analysis (Table 1). Understorey cover density and tree diameter at breast height (DBH) standard deviation (SD) were used as measures of vegetation density and structural variation and were included as continuous fixed effects. The presence of livestock (animals or fresh signs [spoor, dung] observed in the woodland during the study period) and gamebird management inside the woodland boundary represented woodland management, and were both included as dichotomous fixed effects. A patch shape index was calculated and included as a continuous fixed effect to investigate the relationship between woodland shape (edge-to-area ratio) and alarm call rates, given the importance of this variable in the literature.
Sampling period (morning, afternoon or evening) was likely to be an important predictor of alarm call rates due to changes in bird activity throughout the day and was included as a three-level categorical fixed effect. To account for pseudoreplication and seasonal effects on alarm call rates (Fasanella and Fernández 2009), site ID of the focal woodland (n = 32 sites) and sampling day in the month (n = 4 days) were included as random intercepts.
All explanatory variables included in the model above could also affect Eurasian Wren densities. For example, densities might be lower in woodlands with livestock if animals damage potential nest sites, making it difficult to know if livestock affect perceived predation risk or if they cause lower densities. We did not measure densities concurrently with alarm call rates in the 32 woodlands during this study but relative abundance of Eurasian Wrens was quantified in the previous year in a larger sample of 101 woodlands in the same landscapes (Whytock et al. 2017). To examine if Eurasian Wren densities in these landscapes were correlated with the predictor variables of interest, we constructed a generalised linear model with a negative binomial error distribution. Fixed effects replicated those of the previously described alarm call model. Relative abundance was used as the response variable and was measured as the total number of Eurasian Wren registrations from three territory mapping surveys in 2015 (see Methods in Whytock et al. 2017). Based on results in Whytock et al. (2017), we expected to see a strong effect of patch size and little or no effect of the other variables on Eurasian Wren relative abundance. Results confirmed these expectations (Table S1). Although we did attempt to control for patch size during site selection, there was some variation. Given that Eurasian Wren densities were strongly correlated with patch size we therefore included patch size as an offset term in the alarm call model to control for potential differences in densities between sites of different size.
Before fitting the models, we examined bivariate correlations between all predictors to detect multicollinearity (Table S2; supplementary material in Whytock et al. 2017). In the alarm call data (n = 32 sites), the proportion of urban land cover at 500 and 3000 m were highly correlated (r = 0.64), and we retained only urban land cover at 3000 m because it had the strongest univariate relationship with alarm call rates (assessed using binomial GLMMs with the random effects structure and offset of patch area outlined previously).
Continuous predictors were mean centered and scaled by 1 SD, and dichotomous fixed effects were transformed so that 0 became − 1. This allowed effect sizes to be compared directly with those of continuous fixed effects. The final alarm call model was fitted using the glmmTMB R package (Brooks et al. 2017) and the relative abundance GLM was fitted using the glm.nb() function from the MASS package (Venables and Ripley 2002). Pseudo R2 values were calculated using the method given by Nakagawa and Schielzeth 2013. We did not conduct model-selection to avoid omitted variable bias and instead interpreted effect sizes and confidence intervals from the full model.
Results
Alarm calls were detected in 125 (8.15%) of the 10 min audio sections out of a total sample of 1536 (256 h of audio, n = 32 woodlands). Results for the generalised linear mixed effects model (\({\text{R}}^{2}_{\text{marginal}}\) = 0.18, \({\text{R}}^{2}_{\text{conditional}}\) = 0.26) are shown in Table 2. Figure 2 shows the standardised effect sizes and confidence intervals for all predictors used in the analysis.
Caterpillar plot showing standardised coefficient estimates and Wald 95% confidence intervals for all fixed effects in the generalised linear mixed effects model (response variable alarm presence per 10 min audio section). The estimates for the control variable ‘sampling period’ are not shown (see Table 2)
The proportion of agricultural land in the landscape within a 500 m buffer had the strongest positive effect on alarm call probability (Figs. 2, 3a). The mean probability of detecting an alarm call per 10 min almost doubled from 0.12 (0.04–0.29 CI) to 0.23 (0.07–0.54 CI) when the proportion of agricultural land increased from 0.21 to 0.97 within 500 m (the data range). The presence of gamebird management inside the woodland had the strongest negative effect on alarm call probability (Figs. 2, 3b), with the probability of detecting an alarm call per 10 min declining from 0.19 (0.07 – 0.43 CI) in woodlands with no gamebird management to 0.05 (0.01–0.14 CI) with gamebird management.
Alarm call occurrence was also positively correlated with livestock presence, the amount of woodland cover within 500 m, and the amount of urban land cover within 3 km (Fig. 3c,d, e). Effect sizes for the remaining variables were close to zero and had high uncertainty (Fig. 2).
Discussion
Landscape change due primarily to conversion for agriculture threatens species and ecosystems, with forest habitats and species particularly at risk (Haddad et al. 2015). Patterns of species and community responses to habitat fragmentation and loss are well studied but it can be difficult to identify the ecological or behavioural mechanisms that drive these patterns (Dolman 2012). Evidence from studies of multiple taxa show that when individuals perceive an increased risk of predation, this can have negative, cascading effects on individuals and populations (Ripple and Beschta 2004; Cresswell 2008; Dunn et al. 2010; Resetarits and Silberbush 2016). Here, we found that both local management and regional patterns of land-use are correlated with ‘fear’ in fragmented woodlands in agricultural landscapes. Statistical effect sizes suggested that local management and land-use within 500 m were relatively more important than land-cover beyond 500 m.
The strongest predictor of perceived predation risk was the amount of agricultural land (pasture or arable) in the landscape within 500 m of woodland patches. The most likely explanation for this positive relationship is that predator abundance (e.g. corvids, red fox) is higher in landscapes with more agricultural land (Chalfoun et al. 2002) which leads to higher predation rates (and presumably higher perceived predation risk) (Andren 1992; Thompson 2007). However, Whytock et al. 2017 found no evidence to suggest that the amount of agricultural land in the landscape affected woodland bird relative abundance or diversity, and we found the same for Eurasian Wren relative abundance using data from the same year (Table S1). This is counterintuitive and perhaps suggests that perceived predation risk is not correlated with actual predation risk in our study system, or that any negative effect of higher predation pressure or risk related to the amount of agricultural land in the landscape is minimal for the local population.
The relatively local effect of agricultural land on alarm call rates (i.e. < 500 m), could reflect the distance at which individuals begin to perceive a potential threat. Landscapes with a high proportion of agricultural land are also likely to have higher levels of anthropogenic activity (humans, vehicles, dogs), which could cause disturbance and higher perceived predation risk (Rösner et al. 2014). There has been substantial research into the distances at which birds will tolerate a threat before fleeing (flight initiation distances: Weston et al. 2012; Guay et al. 2016), but surprisingly little research has investigated the same phenomenon for alarm calls (i.e. alarm initiation distances) despite the link between the two behaviours (see Fig. 1 in Weston et al. 2012). An interesting direction for future research would be to quantify the distances at which individuals begin to make alarm calls and how this might depend on vegetation structure and the surrounding landscape. This information could be used to inform habitat management, such as creating buffer strips between woodlands and surrounding agricultural land to minimise non-lethal effects of land-use (Guay et al. 2016).
The presence of gamebird management strongly reduced alarm call rates despite our relatively coarse measure of this widespread management practice in the UK. Gamebird management involves the control of common nest predators and there is some evidence to suggest that this can have benign or positive effects on woodland bird abundance (Stoate and Szczur 2001; Draycott et al. 2008), and positively affect nest survival rates of some species (White et al. 2014). Local predator management could therefore explain why perceived predation risk was lower when rearing pens or feeders were present inside woodlands. Nonetheless, it is important to note that gamebird management could also have negative effects on woodland birds. For example, Ring-necked Pheasants Phasianus colchicus could compete with native woodland birds for invertebrate prey, although this has not been quantified directly (Bicknell et al. 2010). Gamebird management can also cause changes in vegetation structure that affects habitat quality for some woodland birds (Draycott et al. 2008). Pringle et al. (2019) showed that gamebird management can cause regional increases in avian predator abundance through the input of additional resources into the environment (prey, carrion), which could have cascading effects on prey species such as woodland birds. Our results suggest that fine-scale management of predators at the local scale (e.g. near feeders or rearing/release pens) might offset this regional effect. Further work is required to determine if gamebird management has benign, net-positive or net-negative effects on woodland birds at individual and population levels (Bicknell et al. 2010).
The presence of livestock had the opposite effect to gamebird management and was correlated with higher alarm call rates. Whytock et al. (2017) found that bird abundance and diversity was lower in woodlands when livestock were present but not because of changes in vegetation structure (contra Martin and Possingham 2005), suggesting a direct disturbance effect. Many woodland bird species in our study areas nest at heights not generally at risk of being trampled, so trampling is unlikely to alter woodland bird densities. Instead, our results suggest that livestock could be creating ‘woodlands of fear’ that are potentially less attractive to colonisers. This mechanism could be tested more explicitly by exploring the relationship between livestock presence in a woodland and the abundance of non-ground nesting species (i.e. those that are not at risk of being trampled), while controlling for other important variables such as patch size, foraging height and prey availability (Martin and Possingham 2005). Alternatively, livestock might indirectly increase predation risk by attracting predators and scavengers (e.g. corvids) into woodland patches and this merits further research.
We did not quantify the demographic or ecological consequences of changes in perceived predation risk. However, evidence from studies of other passerines suggest that higher levels of fear can impact on demographic rates and thus population persistence in a patch, for example by causing lower fecundity or nestling fitness through physiological and behavioural changes (Gentle and Gosler 2001; Dunn et al. 2010 Dudeck et al. 2018). Future research into perceived predation risk using bioacoustic methods should also simultaneously investigate the relationships between environmental factors, perceived predation risk, demographic rates and individual fitness and there is a need to quantify ‘baseline’ alarm call rates in more natural environments.
Other factors not included in our analyses might also play an important role in predicting alarm call rates in Eurasian Wrens. These include individual variation in alarm-initiation distances, differences in the timing of brood stages between study sites, and differences in predator composition between sites, for example. We suggest that future studies attempt to account for these factors, perhaps by combining acoustic data with camera traps to monitor nests directly and to record predator composition in the surrounding area.
The development of inexpensive passive acoustic recorders has made it relatively easy to record alarm calls and other vocal behaviour in passerine birds, and we suggest the following questions are used to guide future research into the relationship between perceived predation risk and environmental factors:
-
1.
How do ‘fear’ levels in agricultural landscapes compare to baseline ‘fear’ levels in landscapes with relatively low levels of human influence (e.g. ancient temperate forest, such as that found in Białowieża National Park, Poland)?
-
2.
Does an increase in perceived predation risk (as measured by alarm call rates) correlate with actual predation risk?
-
3.
Which predators or predator guilds are responsible for causing the most important changes in perceived predation risk as measured by alarm call rates?
-
4.
Do livestock reduce perceived patch quality by creating high levels of fear in agricultural woodlands, or do livestock attract predators into woodlands?
-
5.
Can the negative effects of agriculture in the landscape be ‘buffered’ by modifying woodland edge structure (e.g. buffering hard edges between fields and woodland with semi-natural habitat)?
Conclusion
We have shown that ‘fear’ perceived by Eurasian Wrens in agricultural landscapes is most strongly correlated with land-management activities relatively local to woodland patches. We conclude that, in agricultural landscapes, humans alter the ‘landscape of fear’ through scale-dependent land-management activities, which can both positively and negatively affect perceived predation risk in a common woodland bird. ‘Fear’ is a natural ecological process, but anthropogenic activities could contribute to unnaturally high levels of fear in agricultural landscapes, which could negatively impact on species and ecosystems. Our results suggest that, for a common woodland bird, land managers could reduce fear levels by balancing the prevalence of different management activities at relatively local scales, such as limiting livestock presence inside woodlands or through the use of buffer zones between woodland edges and agricultural fields.
References
Abbey-Lee RN, Kaiser A, Mouchet A, Dingemanse NJ (2015) Immediate and carry-over effects of perceived predation risk on communication behaviour in wild birds. Behav Ecol 27:708–716
Andren H (1992) Corvid density and nest predation in relation to forest fragmentation: a landscape perspective. Ecology 73:794–804
Baker PJ, Molony SE, Stone E, Cuthill IC, Harris S (2008) Cats about town: is predation by free-ranging pet cats Felis catus likely to affect urban bird populations? Ibis 150:86–99
Balmer D, Gillings S, Caffrey B, Swann B, Downie I, Fuller R (2013) Bird Atlas 2007–2011: the breeding and wintering birds of Britain and Ireland. British Trust for Ornithology, Thetford
Batary P, Baldi A (2004) Evidence of an edge effect on avian nest success. Conserv Biol 18:389–400
Beja P, Schindler S, Santana J, Porto M, Morgado R, Moreira F, Pita R, Mira A, Reino L (2014) Predators and livestock reduce bird nest survival in intensive Mediterranean farmland. Eur J Wildl Res 60:249–258
Bellamy PE, Burgess MD, Mallord JW, Cristinacce A, Orsman CJ, Davis T, Grice PV, Charman EC (2018) Nest predation and the influence of habitat structure on nest predation of Wood Warbler Phylloscopus sibilatrix, a ground-nesting forest passerine. J Ornithol 159:493–506
Bicknell J, Smart J, Hoccom D, Amar A, Evans A, Walton P, Knott J, Lodge T (2010) Impacts of non-native gamebird release in the UK: a review. Royal Society for the Protection of Birds, Bedfordshire
Blumstein DT, Mennill DJ, Clemins P, Girod L, Yao K, Patricelli G, Deppe JL, Krakauer AH, Clark C, Cortopassi KA, Hanser SF (2011) Acoustic monitoring in terrestrial environments using microphone arrays: applications, technological considerations and prospectus. J Appl Ecol 48:758–767
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalised linear mixed modeling. R J 9:378–400
Chalfoun AD, Thompson FR III, Ratnaswamy MJ (2002) Nest predators and fragmentation: a review and meta-analysis. Conserv Biol 16:6–318
Cox WA, Thompson FR, Faaborg J (2012) Landscape forest cover and edge effects on songbird nest predation vary by nest predator. Landsc Ecol 27:659–669
Cresswell W (2008) Non-lethal effects of predation in birds. Ibis 150:3–17
Dolman PM (2012) Mechanisms and processes underlying landscape structure effects on bird populations. Birds and habitat: relationships in changing landscapes. Cambridge University Press, UK
Dolman PM, Hinsley SA, Bellamy PE, Watts K (2007) Woodland birds in patchy landscapes: the evidence base for strategic networks. Ibis 149:146–160
Draycott RA, Hoodless AN, Sage RB (2008) Effects of pheasant management on vegetation and birds in lowland woodlands. J Appl Ecol 45:334–341
Dudeck BP, Clinchy M, Allen MC, Zanette LY (2018) Fear affects parental care, which predicts juvenile survival and exacerbates the total cost of fear on demography. Ecology 99:127–135
Dunn JC, Hamer KC, Benton TG (2010) Fear for the family has negative consequences: indirect effects of nest predators on chick growth in a farmland bird. J Appl Ecol 47:994–1002
Fasanella M, Fernández GJ (2009) Alarm calls of the Southern House Wren Troglodytes musculus: variation with nesting stage and predator model. J Ornithol 150:853–863
Ferguson-Lees IJ, Castell R, Leech DI (2009) A field guide to monitoring nests. British Trust for Ornithology, Thetford
Forestry Commission 2013. National Forest Inventory. Available from http://www.forestry.gov.uk/inventory (accessed April 2015)
Garson PJ (1980) The breeding ecology of the Wren in Britain. Bird Study 27:63–72
Gentle LK, Gosler AG (2001) Fat reserves and perceived predation risk in the great tit, Parus major. Proc R Soc London B 268:487–491
Gosler AG, Greenwood JJ, Perrins C (1995) Predation risk and the cost of being fat. Nature 377:621–623
Guay PJ, van Dongen WF, Robinson RW, Blumstein DT, Weston MA (2016) AvianBuffer: an interactive tool for characterising and managing wildlife fear responses. Ambio 45:841–851
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052
Hilton GM, Ruxton GD, Cresswell W (1999) Choice of foraging area with respect to predation risk in redshanks: the effects of weather and predator activity. Oikos 87:295–302
Krebs JR (1980) Optimal foraging, predation risk and territory defence. Ardea 68:83–90
Lahti DC (2001) The “edge effect on nest predation” hypothesis after 20 years. Biol Conserv 99:365–374
Mandema FS, Tinbergen JM, Ens BJ, Bakker JP (2013) Livestock grazing and trampling of birds’ nests: an experiment using artificial nests. J Coast Conserv 17:409–416
Martin TG, Possingham HP (2005) Predicting the impact of livestock grazing on birds using foraging height data. J Appl Ecol 42:400–408
Morton D, Rowland C, Wood C, Meek L, Marston C, Smith G, Wadsworth R, Simpson IC (2011) Final Report for LCM 2007—the new UK Land Cover Map. Countryside Survey Technical Report No. 11/07
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalised linear mixed-effects models. Methods Ecol Evol 4:133–142
Platzen D, Magrath RD (2004) Parental alarm calls suppress nestling vocalization. Proc R Soc London 271(1545):1271–1276
Pringle H, Wilson M, Calladine J, Siriwardena G (2019) Associations between gamebird releases and generalist predators. J Appl Ecol. https://doi.org/10.1111/1365-2664.13451
Resetarits WJ, Silberbush A (2016) Local contagion and regional compression: habitat selection drives spatially explicit, multiscale dynamics of colonisation in experimental metacommunities. Ecol Lett 19:191–200
Ripple WJ, Beschta RL (2004) Wolves and the ecology of fear: can predation risk structure ecosystems? Bioscience 54:755–766
Rösner S, Mussard-Forster E, Lorenc T, Müller J (2014) Recreation shapes a “landscape of fear” for a threatened forest bird species in Central Europe. Landsc Ecol 29:55–66
Stephens SE, Koons DN, Rotella JJ, Willey DW (2003) Effects of habitat fragmentation on avian nesting success: a review of the evidence at multiple spatial scales. Biol Conserv 115:101–110
Stoate C, Szczur J (2001) Could game management have a role in the conservation of farmland passerines? A case study from a Leicestershire farm. Bird Study 48:279–292
Storch I, Woitke E, Krieger S (2005) Landscape-scale edge effect in predation risk in forest-farmland mosaics of central Europe. Landsc Ecol 20:927–940
Templeton CN, Greene E, Davis K (2005) Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308:1934–1937
Thompson FR III (2007) Factors affecting nest predation on forest songbirds in North America. Ibis 149:98–109
Thompson FR III, Donovan TM, DeGraaf RM, Faaborg J, Robinson SK (2002) A multi-scale perspective of the effects of forest fragmentation on birds in eastern forests. Stud Avian Biol 24:8–19
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Watts K, Fuentes-Montemayor E, Macgregor NA, Peredo-Alvarez V, Ferryman M, Bellamy C, Brown N, Park KJ (2016) Using historical woodland creation to construct a long-term, large-scale natural experiment: the WrEN project. Ecol Evol 6:3012–3025
Wesołowski T (1983) The breeding ecology and behaviour of Wrens Troglodytes under primaeval and secondary conditions. Ibis 125:499–515
Weston MA, McLeod EM, Blumstein DT, Guay PJ (2012) A review of flight-initiation distances and their application to managing disturbance to Australian birds. Emu-Austral Ornithol 112:269–286
White PJ, Stoate C, Szczur J, Norris K (2014) Predator reduction with habitat management can improve songbird nest success. J Wildl Manag 78:402–412
Whytock RC, Christie J (2017) Solo: an open source, customizable and inexpensive audio recorder for bioacoustic research. Methods Ecol Evol 8:308–312
Whytock RC, Fuentes-Montemayor E, Watts K, Barbosa de Andrade P, Whytock RT, French P, Macgregor NA, Park KJ (2017) Bird community responses to habitat creation in a long-term, large-scale natural experiment. Conserv Biol. https://doi.org/10.1111/cobi.12983
Zanette LY, White AF, Allen MC, Clinchy M (2011) Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334:1398–1401
Acknowledgements
We are grateful to landowners for permitting access to the study sites. We thank Mark Whittingham, Luc Bussiere and two anonymous reviewers for their valuable comments on earlier versions of the manuscript.
Funding
This work was supported by the IAPETUS DTP via the Natural Environment Research Council (grant number NE/L002590/1), the National Forest Company and Forest Research. The Woodland Creation and Ecological Networks project has been funded by the University of Stirling, Natural England, Forestry Commission, Scottish Natural Heritage, the Department for the Environment, Food and Rural Affairs, the National Forest Company, Forest Research, the Woodland Trust and Tarmac Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whytock, R.C., Fuentes-Montemayor, E., Watts, K. et al. Regional land-use and local management create scale-dependent ‘landscapes of fear’ for a common woodland bird. Landscape Ecol 35, 607–620 (2020). https://doi.org/10.1007/s10980-019-00965-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00965-x