Abstract
Climate warming can induce the encroachment of shrubs and may trigger treeline dynamics. However, the responses of shrubs and trees to climate change may be modulated by other environmental drivers such as land-use change and biological interactions. The European Alps are one of the three areas experiencing the most intense warming globally in the twentieth century. We analyse, through a multidisciplinary approach, the shrub and tree encroachment at the Stelvio Pass (Italian Alps) focusing on three target species (Rhododendron ferrugineum, Larix decidua, Pinus mugo) to reconstruct their dynamics and assess which drivers (climate change, land-use change, biological interactions provided by shrub facilitation) promoted their ingression. Shrub colonization started in 1867, in coincidence with the end of the Little Ice Age. Tree recruitment started since 1960 for P. mugo and 1972 for L. decidua and correlated strongly with air warming and shortening of the snow cover duration. Climate (air temperature, snow cover) exhibited the highest correlation with shrub and tree dynamics both during the period of recruitment and in the following and preceding 5-year period. Air warming appeared to be crucial for tree ingression and persistence. Land-use change was not related to shrub encroachment, and only weakly to tree recruitment. Both the correlation analysis as well as the patterns of recruitment highlighted that shrubs were characterized by different ecological requirements from trees. There was not a “nurse effect” of shrubs on trees, and this biotic interaction changed with the developmental stage of the involved species, being lowest for seedlings and highest for adults, requiring further investigations. Future scenarios of climate change indicate a further and intense warming, and our data show that it is likely that shrub and tree encroachment will proceed, with relevant consequences on the extremely vulnerable high-elevation alpine ecosystems.
Similar content being viewed by others
Highlights
-
Shrub ingression in the European Alps started at the end of the Little Ice Age (1860s)
-
Persistence and intensification of warming triggered tree ingression since 1970
-
Land-use change or plant biotic interactions did not drive shrub ingression
-
Biotic interactions with shrubs changed with the developmental stage of the tree species
Introduction
In the last decades, increasing evidence of the impacts of climate change on terrestrial ecosystems has been observed across our planet, affecting them at different levels of ecological organization, with high-latitude and high-elevation regions being particularly sensitive (Walthers 2003; IPCC 2014).
The upward migration and areal expansion of shrubs are two of the most evident impacts of climate change worldwide (Elmendorf and others 2012a, b; Myers-Smith and Hik 2018) and are able to induce significant feedbacks to climate through changes in the surface energy balance, carbon balance and hydrology (Sturm and others 2005). These impacts are well evident in the European Alps (for example, Lenoir and others 2008; Gottfried and others 2012), one of the three areas of the planet experiencing the most intense warming in the twentieth century, in particular in the period 1950–2000 (Böhm and others 2001; Auer and others 2007).
Climate warming may also impact treeline dynamics, promoting upslope advances and/or treeline infilling (for example, Lenoir and others 2008; Camarero and others 2017). The treeline is defined as the boundary of the trees taller than 2–3 m growing at the highest altitude (treeline elevation) (Körner 2003, 2012), and, at global scale, its position is considered temperature sensitive and, hence, expected to respond to climate warming (Körner and Paulsen 2004; Körner 2003, 2012; Holtmeier and Broll 2017). However, at regional and local scales, the responses of both shrubs and trees to climate change may be modulated by other environmental drivers, such as historical land-use change (Gehrig-Fasel and others 2007; Komac and others 2013), disturbance (Leonelli and others 2016) and biological interactions (Angers-Blondin and others 2018; Frei and others 2018). In particular, in the harsh alpine environment, facilitative or competitive interactions between trees and shrubs (Angers-Blondin and others 2018; Frei and others 2018) are fundamental to understand past successional stages and dynamics and to predict the future rates of encroachment (Pornon and Doche 1995; Batllori and Gutiérrez 2008; Holmgren and others 2015).
The assessment of the spatial and temporal trends and the quantification of shrub encroachment and population development are rare and localized both in alpine and arctic areas (for example, Anthelme and others 2007; Cannone and others 2007; Elmendorf and others 2012a; Frost and Epstein 2014; Pornaro and others 2017), and mainly obtained as a result of ecological monitoring of shrub cover. Past treeline dynamics have been documented by several studies, both through the combination of vegetation mapping and dendrochronological analyses, as well as through manipulation experiments. The patterns of tree recruitment are influenced by propagule pressure and the differing invasibility of the different grassland communities, as observed for Pinus mugo in the subalpine and alpine grasslands in the European Alps (Dullinger and others 2003). The occurrence of synchronous recruitment trends of different populations of the same species could be interpreted as the result of regional climatic factors modulating tree line structure and dynamics, as for Pinus uncinata in the Pyrenees (Batllori and Gutiérrez 2008; Batllori and others 2009). In the central Italian Alps, an ongoing treeline upward shift was recently documented with rate of up to 2.6 m/y in the period 2000–2009, indicating that climatic conditions related to the ongoing air temperature rise in the study region will likely enhance the treeline shift (Leonelli and others 2016). Transplant experiments have shown that there was better seedling growth at the treeline compared to the forest, and that shrub occurrence prevented seedling winter damage and herbivory (Grau and others 2013). To our knowledge, few studies have quantified shrub and treeline dynamics in the same location, or examined the influence of biotic and abiotic interactions between shrubs and trees on treeline dynamics.
To investigate the relation between population dynamics of woody species and environmental drivers at the treeline ecotone, a multidisciplinary approach involving the combination of different methods (for example, ecological monitoring, remote sensing, repeat photographs, satellite imagery, dendroecology) and based on several lines of evidence (for example, coverage, growth, reproduction, shrubline, recruitment year) is recommended (Myers-Smith and Hik, 2018). In particular, dendrochronology is a valuable tool and a standardized method allowing us to reconstruct the population dynamics of shrubs and trees and their recruitment rates (for example, Batllori and Gutiérrez 2008; Myers-Smith and others 2015).
For a high-elevation site (Stelvio Pass) above the treeline (> 2200 m a.s.l.) located in the central Italian Alps, the availability of a detailed phytosociological mapping and of phytosociological relevés elaborated in 1953 (Giacomini and Pignatti 1955) allowed us to document and quantify the vegetation changes occurred. In particular, since 1950 a striking vegetation change occurred, with upward displacement of several vegetation communities and rapid shrub expansion (mainly sustained by Rhododendron ferrugineum L.), contemporary to an observed significant warming of air temperature and a decrease in snow cover thickness and duration (Cannone and others 2007). At this site, the only anthropogenic land use was extensive summer pasturing, as documented by the historical archives, allowing us to exclude land-use change as main driver of the observed vegetation changes (Cannone and others 2007; Cannone and Pignatti 2014).
Here, we analyse, through a multidisciplinary approach, shrub and tree encroachment that occurred at the Stelvio Pass site focusing on three target species: R. ferrugineum (RF), Larix decidua Mill. (LD) and Pinus mugo Turra (PM). We hypothesize that:
-
(1)
Given the existing evidence that R. ferrugineum is sensitive to warming (Cannone and others 2007), we expect that encroachment and upward migration of R. ferrugineum (started in 1867, in coincidence with the end of the Little Ice Age (LIA), as demonstrated by the dendrochronological data) were driven primarily by climate change (air temperature and snow);
-
(2)
Due to the widespread sensitivity of treelines to temperature, we expect that the occurrence of persistent climate warming, combined with decreasing grazing since the 1870s, promoted the encroachment and upward migration of tree species within the high alpine tundra.
Moreover, our analyses aimed to highlight that shrubs did not facilitate tree encroachment through a “nurse” effect and that the relation between shrubs and trees was species-specific and changed depending on the developmental stage of trees.
For these aims, we analysed through dendrochronological methods combined with vegetation mapping, the spatial and temporal dynamics of the selected target shrub and tree species, in order to obtain their recruitment curves and identify their relation with climate and other environmental drivers (for example, land-use change, biological interactions). This case study provides a unique opportunity given the availability of historical data concerning climate (including snow) (since 1875), grazing (since 1800), and a detailed vegetation dynamics (since 1867 for dendrochronology). Despite the fact that our data cannot allow replication, our results represent a template of the dynamics of shrub and tree encroachment occurring at high-elevation sites in response to recent climate and land-use change.
Materials and Methods
Study Area
The study area is located in the Italian central Alps, close to the Stelvio Pass (46° 31’ 43.1″ N 10° 27’ 11.2″ E; elevation 2230–2750 m a.s.l.), a high alpine site in the Stelvio National Park (Figure 1).
Climatic data (period 1978–2015) from the nearest available meteorological station at Cancano (46° 31′ 02.2″ N, 10°19′ 14.7″ E, 1948 m asl, 9 km to E–SE) indicate a mean annual air temperature of + 3.3 ± .75°C, with January as the coldest and July as the warmest months (means = − 5.2 ± 1.8°C and + 12.2 ± 1.6°C, respectively). Mean annual precipitation sum is 810 mm, 56% of which falls between May and September. Snow can fall at any time, but lays continuously for 6 months, from mid-November to May.
The bedrock is mainly siliceous with some outcrop of calcareous rock in the south-west sector of the study area. Permafrost is sporadic at elevations between 2400 m and 2700 m (Cannone and others 2003), and discontinuous above (Guglielmin and Siletto 2000). Nevertheless, 1 km east from the study area at 3000 m, the permafrost thickness exceeds 200 m (Guglielmin and others 2018).
Vegetation is a mosaic of habitat types of the subalpine and alpine belts, including chionophylous alpine shrubs (target species Rhododendron ferrugineum), alpine dwarf shrubs of the wind-swept areas, alpine grasslands, snowbeds, pioneer vegetation and barren ground.
The tree species line (Körner and Paulsen 2004) in this area is formed mainly by seedlings and saplings of Larix decidua Mill. and Pinus mugo Turra, with few individuals of Picea excelsa Link. Vegetation maps of the study site are available for year 1953 (Giacomini and Pignatti 1955) and 2003 (Cannone and others 2007).
At the elevation of our study site, the only anthropogenic land use is extensive summer pasturing. Transhumance in this area is known since 1800 and practiced during the summer when cattle herds are taken to the highland, while in autumn they are brought back to the lowland.
Field Survey and Sampling Design
In the field, a detailed survey to map the spatial distribution of R. ferrugineum shrubs and of tree species occurring in the study area was performed in 2013 at 1:2000 scale, using a GPS Garmin eTrex30 device with a precision of ± 3 m. All visible (taller than 5 cm) shrub and tree individuals (including dead individuals) were censed. For all tree species individuals (926) and for a subset of shrubs (494 individuals), the following variables were recorded in the field: geographic position (Lat N, Long E, WGS84, dd.dddd format), plant height (cm), longest canopy diameter (canD1; cm), second canopy diameter (canD2; cm –measured perpendicular to canD1) and basal stem diameter (SBD; mm) measured near the stem–root interface. We differentiated between seedlings (height < 50 cm), saplings (height 51–130 cm) and adults (height > 131 cm) (Fidej and others 2016). These standard parameters were recorded as they are usually employed to analyse the morphological and demographic structure of shrub (that is, Batllori and Gutiérrez 2008; Leonelli and others 2016) and tree populations (that is, Pornon and Doche 1995; Myers-Smith and others 2015).
Shrub and Tree Age Determination
Once the mapping was completed, sampling was performed according to Myers-Smith and others (2015) for shrubs and to Dofour-Tremblay and others (2012) for trees. In particular, based on the results of the morphometric analyses of shrubs and trees, and considering that one of the aims of our work was the assessment of the onset of shrub and tree colonization with relation to climate, according to Myers-Smith and others (2015), we performed a subjective sampling of larger and older individuals. Moreover, to understand the patterns of development of the shrub and tree populations and determine age distributions across our study site, we also performed a random sampling (Myers-Smith and others 2015). As dendrochronological sampling is destructive, we selected a subset of individuals representative of the observed morphometrical, topographical and altitudinal variability of each species. Totally, 46 individuals of R. ferrugineum and 105 tree species individuals (44 of P. mugo, 61 of L. decidua) were sampled in 2013 for age determination, performed according to standard dendrochronological protocols. Trees with a basal diameter above 5 cm were cored at the lowest possible level (soil level) with a 4.35 mm Pressler core borer (Haglof, Sweden), whereas a full disc was taken at the root collar for smaller individuals (that is, Dofour-Tremblay and others 2012). In case of polycormic individuals, the stem with the biggest basal diameter was selected for sampling (that is, Caccianiga and Compostella 2012). To provide a very accurate age determination, the serial-sectioning technique was adopted for both shrub and trees species (Myers-Smith and others 2015; Leonelli and others 2016).
After collection, all samples were dried and finely sanded (Myers-Smith and others 2015). Ring number and ring width were measured with a measuring stage Lintab 6 and the time series analysis program TSAPWin ver.4.64 (Rinntech), with an accuracy of .01 mm. Mean ring-width series from discs and cores taken at different stem length were compared and cross-dated using the Cross-Date Index (CDI) values (TSAPWin software) and visual inspection (that is, Schmidt and others 2006). For the selected tree species, the recruitment year corresponded to the start of the individual growth. Differently, for R. ferrugineum, the plant age may reflect either the germination date or the date of ramet formation (that is, vegetatively produced plant of the genet that is actually or potentially independent; Pornon and others 1997) because of the ability of the species to perform clonal growth.
For further computations, the recruitment dates of both trees and shrubs were classified by time intervals (for example, 5 years) (that is, Pornon and Doche 1995; Pasche and others 2004; Gamache and Payette 2005; Dofour-Tremblay and others 2012).
Data Elaboration and Link with Climatic and Environmental Drivers
The distribution patterns of the three target species were analysed in relation with topography. For this aim, the 5x5 m cell-size digital elevation model (Geoportale Regione Lombardia, coordinate reference system WGS84/UTM32) was used to extract the following topographical variables: elevation (m asl), slope (degree) and aspect (°N).
For the analysis of the relation between plant dynamics and climate, we used data recorded at AWS Segl-Maria (Swiss National Basic Climatological Network; station code: SIA; 46° 25′ 56.388′′ N, 9° 45′ 44.352′′ E, 1804 m asl). It was the closest AWS to our study site that provides the longest climatic data series (1864–2015) and that had strong linear regression with temperature (R2 = .99, p < .001) and precipitation (R2 = .81, p < .001) recorded at AWS Cancano (located 50 km far from Segl-Maria and 9 km far from the study site).
We analysed the climatic data of target months representative of spring (for example, April), summer (for example, July) and autumn (for example, November) to show that the climate warming did not have a linear trend with time since 1865 (Segl-Maria AWS data), and then, we provided a focus on the period since 1980 s’ to emphasize the more pronounced recent warming.
To assess whether land-use change, with special reference to grazing, could have driven the observed encroachment of shrubs and trees, we analysed the changes of the livestock population of Alpe Braulio farm obtaining all the available historical data for the period 1800–1993 from the official historical archives of the Mountain Community of Alta Valtellina, Fondazione Fojanini and from literature (Giacomini and Pignatti 1955). From 1993 to present, detailed data on grazing were achieved from personal communication with the pasture holder and local shepherd. Detailed data on grazing are reported in Table 2 Supplementary Materials, with the indication of the grazing rate used for each 5-year period for the statistical analyses.
The climate time series of Segl-Maria AWS and Cancano AWS averaged by five-year periods for the whole study period (since 1875) were used to perform a product-moment correlation (Pearson correlation coefficient, r) to test, on a five-year scale, correspondence between shrub and tree recruitment, climate data (that is, Gamache and Payette 2005; Francon and others 2017), grazing pressure and interaction with the recruitment of the other shrub or tree species. In addition, we performed a further correlation analysis on the period after 1970 because all data (climate, grazing, shrub and tree recruitment) were available, allowing us to investigate the role of all drivers (climate change, land-use change, biological interactions) on the single target species.
As seed germination may require long time periods (Bernareggi and others 2015) and does not ensure individual survival and persistence (Angers-Blondin and others 2018; Frei and others 2018), we analysed the relationship between climate and the five years before (5-yrs_t − 5) and after (5-yrs_t + 5) recruitment.
To focus on the potential nurse effect likely provided by the relation between shrubs and trees, for each mapped and measured tree individuals, we described the physionomical and ecological characteristics of the surrounding vegetation, through the map available for year 2013 (unpublished) and the field survey. For this purpose, we used three main vegetation categories: (1) shrub communities (VR) = acidophilous shrub communities dominated by R. ferrugineum (All. Rhododendro ferruginei-Vaccinion myrtilli A.Schnyd. 1930); (2) dwarf shrub communities (LC) = silicicolous, alpine heaths dominated by nano-phanerophytes (that is, Kalmia procumbens) and lichens (All. Loiseleurio procumbentis-Vaccinion microphylli Br.–Bl. in Br.–Bl. & Jenny 1926); (3) other (herbaceous) communities = areas without shrub dominance, mainly characterized by subalpine and alpine grasslands. Because of the dependence between the colonization success and invasion susceptibility of different vegetation types and the presence of sheltered sites for seed germination (that is, Dullinger and others 2003; Holmgren and others 2015), we obtained the number and the frequency of tree individuals that had germinated in shrubs, dwarf shrubs or other areas. In addition, beyond the location of each individual tree in relation to the community type, we added the data recorded at the microscale in the field on the distance of each individual tree from the closest individual of R. ferrugineum and analysed quantitatively this relation for each individual tree species and for each developmental stage (seedlings, saplings and adults).
Moreover, repeated analyses of variance (ANOVA) and Student’s t test were applied to identify significant differences in age, ring width, stem basal diameter, plant height and longest canopy diameter of trees species between the ecological categories and, therefore, to assess the occurrence of a “nurse effect” of shrubs communities on trees establishment.
All statistical analyses were performed using the package STATSOFT10©.
Results
Spatial Distribution Patterns of Shrubs and Trees
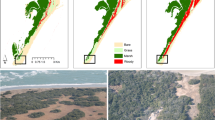
In 2013, in the study area, about 49.3 ha were occupied by shrub communities (8.5% of the study area), and, of this area, 9.8 ha (1.7%) were dominated by R. ferrugineum.
R. ferrugineum occurred across an elevation range between 2306 and 2459 m, with almost half of the population (47.9%) being concentrated between 2350 and 2399 m, on steep (20–29°: 49.3%) or very steep (30–39°: 42.8%) and mainly north-west (61.5%) facing slopes (Table 1, Figure S1a Supplementary Information). Few single and scattered individuals of R. ferrugineum were found up to 2576 m (not sampled).
Among trees, totally 494 individuals were recorded (Table 1): 147 of Pinus mugo (PM) (85 seedlings, 58 saplings, 4 adults) and 347 of Larix decidua (LD) (102 seedlings, 227 saplings, 18 adults). Overall, P. mugo was distributed between 2263 and 2628 m, but with a preference for an elevation range between 2338 and 2425 m on steep (20–29°: 43.5%) or very steep (30–39°: 42.2%) north-west (52.4%) facing slopes (Table 1, Figure S1b Supplementary Information). L. decidua occurred across an elevation range between 2259 and 2597 m, with a prevailing location between 2376 and 2409 m, on steep (20–29°: 31.7%) or very steep (30–39°: 59.4%) north-west (47.0%) and west (42.1%) facing slopes (Table 1, Figure S1c Supplementary Information).
The spatial distribution of all the analysed shrub and tree species was similar across the study area (Figure S2 and Figure S3 Supplementary Information), with an initial colonization occurring in the south-western part of the study area, closer to the Braulio Valley, being the source of the new individuals, and a further expansion northward towards the Stelvio Pass and the higher elevation sites. From the first individuals, the colonization proceeded forming small patches at the same elevation and only after proceeded upwards. This strategy was adopted by all species.
Climate and Land Use
Air warming started since 1875 although the analysis of the seasonal trends (spring, summer, autumn) shown the lack of a linear trend with time (Figure 2A–C). In particular, we observed similar patterns comparing spring (represented by April as template month) and autumn (for example, November), but slightly different for summer (for example, July) (Figure 2A–C). In all cases, there were alternating periods of warming, followed by cooling. Since 1980 (up to 2015), spring and autumn exhibited a statistically significant and continuous warming, with spring showing a rate (ß = + .077, R = .57, p < .001) that largely exceeded autumn warming (ß = + .05, R = .34, p = .04). Summer (Figure 2B) was characterized by some periods with intensive warming (such as between 1980 and 1995 and after 2003), but without any statistically significant trend. Similarly, to spring and autumn, also the mean annual air temperature exhibited a statistically significant warming trend only in the period 1980–2015 (Figure 2D) (ß = + .054, R 0.7, p < .05).
Trends of air temperature in A April, B July, C November (as target months representing different seasons) since 1865 and D on the MAAT (mean annual air temperature) since 1975 to emphasize the increasing warming trend of the last decades (as shown by the linear regression equation). Data from the Segl-Maria AWS. The red square indicates the period of the maximum recruitment and tree ingression.
As not only climate but also grazing and land-use change may affect treeline dynamics and tree encroachment, the changes of the livestock population of Alpe Braulio farm were reconstructed since 1842. The largest livestock population size was recorded in 1842 with 1937 individuals (448 bovine, 864 ovine, 625 equine) and slowly decreased until 1950, when the livestock included only cows and sheep (Figure 3). In the period 1950–1978, there was a further decrease and the complete disappearance of sheep. Since 1978 to nowadays, the grazing pressure remained constant. For what concerns the large herbivores, since 1999, ibex (Capra ibex L.) and chamois (Rupicapra rupicapra rupicapra L.) did not show any significant trend in the number of individuals (data not shown, Stelvio National Park personal communication), whereas other information on small herbivores, such as rodents or mountain hares, is not available.
Shrub and Tree Demography
Concerning the age and demography of the sampled individuals (46 R. ferrugineum; 44 P. mugo; 61 L. decidua), R. ferrugineum exhibited the oldest age ranging between 145 and 12 years, corresponding to the start of recruitment in 1867. It had a low and discontinuous recruitment rate until the period 1920–1924 (Figure 4A), whereas the first appreciable increases were detected in the periods 1925–1929 (+ 8.7%) and 1930–1934 (+ 6.5%). The highest recruitment rates of R. ferrugineum were recorded during the periods 1975–1979 (+ 15.2%) and 1980–1984 (+ 10.5%).
Tree ingression occurred later than shrub establishment (Figure 4B, C). The recruitment of P. mugo started in 1960 (with measured ages ranging between 52 and 4 years, corresponding to the first ingression in the year 1960), while L. decidua started to colonize the area only later in 1972 (with an age ranging between 40 and 2 years, corresponding to the first ingression in the year 1972). Since the first stem initiation, for both species, we observed an exponential increase in the recruitment coupled with a strong increase in the air temperature (Figure 4B, C), with the maximum recruitment rates recorded for P. mugo during the periods 1995–1999 (+ 20.5%) and 2000–2004 (50.0%) and for L. decidua during the periods 1990–1994 (+ 21.3%) and 2000–2004 (+ 39.4%). The average population age and the main recruitment events did not differ between the tree species.
Drivers of Shrub and Tree Recruitment
The correlation analysis allowed us to assess which environmental or biological driver (climate, grazing, biological interaction provided by the recruitment of the other species) was more strictly related to the observed recruitment of our target shrub and tree species. Air temperature exhibited the highest correlation with the recruitment of all species, but there were important differences, indicating an opposite relationship with climate of R. ferrugineum compared to L. decidua and P. mugo (Table 2). The air temperature in April influenced all species, although with opposite trends in R. ferrugineum (favoured by a shorter growing season and colder April) vs the two tree species (taking advantage of a spring advance provided by shorter snow cover duration and warmer April). The ingression of shrub and trees was linked also to the climatic conditions of both the five years before and after the recruitment (Table 2) (with the exception of P. mugo for the five years before the recruitment), confirming opposite trends for the shrub compared to the trees. Indeed, during the decade before the recruitment, R. ferrugineum was favoured by a cooler and moister August and a cooler MAAT, while the opposite was observed for the trees, taking advantage also from a warmer July (for L. decidua) and June (for P. mugo) (Table 2). R. ferrugineum was positively influenced by the snow cover, in particular during spring (April and May), when snow likely provided protection against spring frost events. Trees were positively related to total precipitation in November (as snow), when snow likely provided shelter from the cold winter temperatures. The key role of air temperature during spring (April but also May) before, during and after the recruitment period was evident especially for R. ferrugineum (Table 2). The correlation analysis also showed that there was not any relationship between R. ferrugineum and tree recruitment. However, recruitment patterns of the two tree species were highly similar, probably due to their similar ecological requirements, as outlined by their relationship with climatic data.
Interestingly, grazing did not exert any effect, neither during the recruitment period, nor during the 5-year periods before or after on R. ferrugineum. A slight negative relationship with grazing was observed for both trees when considering the whole study period (1875–2015).
However, the correlation analysis performed on the more recent period (from 1970 to present) showed that the recruitment patterns of trees exhibited relationships exclusively with the climatic parameters (Table 2).
Shrub-Tree Interaction: Nurse Effect?
Our data indicated opposite temporal trends between the recruitment of R. ferrugineum vs. L. decidua and P. mugo and also a correlation with different climatic parameters, in particular with respect to the relationship with snow cover (Table 2). The spatial distribution patterns of seedlings and saplings of L. decidua and P. mugo in relation to the community type (shrub (VR) vs. dwarf shrub (LC) vs. open herbaceous communities (grasslands)) highlighted a species-specific difference in the areas of recruitment preference (Figure 5). Indeed, the majority of seedlings of both L. decidua and P. mugo preferred open herbaceous communities without shrub dominance, followed by dwarf shrubs (Figure 5A, C), whereas the saplings and, above all, the adults exhibited a preferential location within shrub communities (> 43% for saplings; > 69% for adults) for both tree species (Figure 5A, C). Concerning the distance of tree individuals from the nearest individuals of R. ferrugineum (Figure 5B, D), the preference of seedlings for shrub-free sites is strengthened as it accounts for more than 70% of the whole population for P. mugo and more than 50% for L. decidua. For saplings, both species show a slight preference for microsite above or close to R. ferrugineum individuals (≤ 2 m or inside), whereas adults of both species exhibited a clear preference (especially for P. mugo) for a location inside the shrub individuals (Figure 5B, D). These data demonstrate quantitatively that an individual tree can have a very different relationship with shrubs depending on its life stage.
A, C Location of tree species individuals (% population) (LD = L. decidua; PM = P. mugo) in different community types and micro-habitat conditions comparing seedlings, saplings and adults (recorded on year 2013); B, D distance of the tree species individuals from individuals of the shrub Rhododendron ferrugineum comparing seedlings, saplings and adults. Legend: shrubs = Vaccinium-Rhododendron (“VR”, R. ferrugineum dominance, shrubs); dwarf shrubs = Loiseleurio-Cetrarietum (“LC”, Kalmia procumbens dominance, dwarf shrubs); grasslands = plant communities and/or ecosystems without shrubs (“others”); seedlings = height < 50 cm; saplings = height 51-130 cm, adults = height > 131 cm; inside_0m = located above a R. ferrugineum individual; close ≤ 2 m = located within a 2 m distance; far > 2 m = located at more than 2 m; RFabsent = located where R. ferrugineum is absent in a ray of 5 m.
Shrub influence on tree establishment could also be assessed through the analysis of the variability of the tree morphological characteristics (age, ring width, plant height, stem basal diameter and canopy diameter of trees species as proxies of favourable growing conditions) between the different ecological categories. The oldest individuals of P. mugo were found in shrub communities (VR); the same response was found for L. decidua (Figure 6D), although there was not any statistically significant difference in maximum tree age depending on the community type. For both tree species, all morphological measurements indicated higher values in areas with shrub dominance compared to shrub-free areas, with these differences being statistically significant (ANOVA, Student’s t test; Figs. 6A–C). Indeed, the prevailing location of older individuals was within shrub-dominated communities than in grasslands and closed or growing inside shrubs (see Figure 5).
A Stem basal diameter (SBD, cm); B plant height (height, cm); C longest canopy diameter (canD1, cm) and D plant age (age, years) of tree species (PM = P. mugo; LD = L. decidua) species, under different micro-habitat conditions (recorder on year 2013). VR = Vaccinium-rhododendron (R. ferrugineum dominance, shrubs); LC = Loiseleurio-cetrarietum (Kalmia procumbens dominance, dwarf shrubs); others = grasslands (plant communities and/or ecosystems without shrubs). Line = median value; boxes = 25–75 percentiles; whiskers = min/max values. Different letters mean significant differences at the intra-specific level, as tested by Student’s t-test.
Discussion
Climate as Driver of Shrub and Tree Ingression and Further Encroachment
Shrub encroachment and migration to higher latitudes and/or elevations have been documented worldwide, from the high-latitude alpine tundra (Dofour-Tremblay and others 2012; Frost and Epstein 2014; Büntgen and others 2015; Myers-Smith and others 2015; Myers-Smith and Hik 2018) to the mid-latitude mountain ranges (Anthelme and others 2007; Cannone and others 2007) as a consequence of recent climate warming.
The European Alps have been affected as well, with the appearance of species and communities from the lower altitudinal belts and upward displacement of shrublands (Pornon and Doche 1995; Cannone and others 2007; Cannone and Pignatti 2014), but still few studies have focused on the relation of shrub and tree population dynamics and implemented the knowledge of this process also for the alpine range.
In the study area, Guglielmin and others (2018) provided a 500-year reconstruction of the ground surface temperature using a 200 m borehole (Stelvio Share borehole). According to this reconstruction, here the Little Ice Age (LIA) started in AD 1560 and ended in AD 1860, with three main negative temperature peaks at AD 1570–1600, 1685–1700 and 1790–1820. The palaeoclimatic reconstruction indicated also the onset of a progressive warming starting after the end of the LIA (Guglielmin and others 2018). Our dendrochronological dating of the oldest R. ferrugineum individuals occurring in the study area indicates that shrub ingression started in 1867, only few years after the end of the LIA as recorded by climatological and geological data (Guglielmin and others 2018). The time coincidence of these two events (end of LIA and shrub ingression) allows us to hypothesize that the shrub ingression was promoted by the climatic amelioration provided by the end of LIA. We also assessed the records of maximum longevity of the three target species selected for this study (202 years for R. ferrugineum, 672 years for L. decidua and 360 years for P. mugo, Table S1 Supplementary Materials) to be sure that our dating represented the species dynamics and not an artefact due to the species’ longevity constraints. The lack of R. ferrugineum individuals older than our oldest individual (146 years old in 2013) (as well as of older tree individuals of those recorded by our data) was not due to longevity constraints, but to the fact that shrubs effectively did not colonize the site before 1867 (and trees did not colonize the site before the second half of the century). A further confirmation of our hypothesis was the observation of similar evidences of post-LIA shrub colonization reported in northern Canada and Greenland (that is, Boulanger-Lapointe and others 2014), where the Arctic willow (Salix arctica) colonized some semi-desert ecosystems during the LIA (AD 1500–1850), with a peak of recruitment at the end of this cold period.
From the first R. ferrugineum establishment up to 1920, there was a low and discontinuous recruitment rate, combined with a general decrease in the seasonal anomalies of temperature (Figure 4). Afterwards, the most important colonization period was in 1975–1984. Our results emphasize that the highest recruitment of R. ferrugineum is highly correlated with the snow cover, in particular with the total number of days with snow cover as well as to April temperature, being favoured by colder April with prolonged snow cover occurrence. These findings are in agreement with the ecological requirements of this species, requiring the snow thermal protection from winter and spring frost events (Neuner 2014) and then providing further water availability during snow melting (Mayr and others 2010). Indeed, during the period 1975–1984, the snow depth and duration were higher compared to the previous and the future periods (for example, Beniston 2012; Giaccone and others 2015), which may have positively affected the growth of R. ferrugineum, as this species is particularly dependent on snow to prevent plant species leaves, buds and roots from freezing and to allow a more efficient photosynthesis at the start of the growing season (for example, Neuner 1999, 2014). Concerning other drivers potentially involved in the observed shrub dynamics, our analyses allowed us to exclude a relationship between shrub ingression and grazing, despite the decrease in livestock size that occurred until the year 1978. The lack of any correlation between shrub encroachment and grazing (as proxy of land-use change) was confirmed by analysing both the whole period (1875–2015) and the period post-1970 (Table 2). Our data are in agreement also with the fact that the toxicity of R. ferrugineum may lead to a low sensitivity of this species to the livestock pressure (Louis and others 2010).
The reduction of R. ferrugineum recruitment observed since the 1980 s’ could be explained in different ways. First, considering the positive relation of R. ferrugineum with the snow depth regime, the reduction of snowfall that occurred especially since the 1980 s’ (Cannone and others 2007; Scherrer and others 2013) provided less protection against freezing damage and could be one of the drivers of the reduced recruitment of the more recent years. Moreover, a thinner snow cover provided less water supply with the consequent potential increase in summer drought, a factor particularly detrimental for the R. ferrugineum seedling survival (Mayr and others 2010; Fernàndez-Martínez and others 2016), which may have worsened in recent years (Auer and others 2007). The reduction of shrubs’ recruitment could also depend on the prevalence of vegetative growth over sexual reproduction that is observed at the recruitment stabilizing phase (sensu Pasche and others 2004). This occurs in completely closed stands, when shrub cover increases and prevents seed germination (Pornon and Doche 1995). A last factor which could have contributed to reduced shrub recruitment could be the rarity of safe sites for seed germination and seedling survival, as most of them are already occupied by a close canopy (Pasche and others 2004). However, the reduction in the germination of new shrubs does not necessary mean a reduction in their cover that otherwise may continue to increase thanks to layering and vegetative reproduction (Pornon and Doche 1995).
Therefore, our case study provides further confirmation of the importance of air temperature and snow cover on the shrub encroachment (Myers-Smith and Hik 2018; Sturm and others 2005).
In our study, since the 1960s and more strongly since the 1990s, individuals of P. mugo (first) and L. decidua (second) made their ingression and established at and above the shrub patches and the shrubline (sensu Angers-Blondin and others 2018). Our data also support our second hypothesis that tree recruitment would correspond strongly with seasonal and annual air warming and shortening of snow cover duration (providing an anticipation of spring onset and growing season lengthening), in agreement with other studies from high-elevation and high-latitude environments (that is, Leonelli and others 2016; Batllori and Gutiérrez 2008; Dofour-Tremblay and others 2012). Our data highlight the importance of the persistence of warming and snow cover changes in the years after the first ingression of trees allowing their persistence. Likely, this is a key factor explaining why trees did not show any ingression earlier in the twentieth century, because the warming (especially during the 1950s) did not last long enough, differently from what happened in the last decades. In addition, our analyses indicate, over the whole study period (1875–2015), a slight negative relationship of tree recruitment with grazing, although the contribution of land-use change was quantified to be less than half compared to climate change (Table 2). It is notable that in the recent decades (since 1970), the statistical analyses indicated that grazing had no influence on tree recruitment, while statistically significant relationships were identified with climate.
We observed opposing recruitment trends in R. ferrugineum compared to L. decidua and P. mugo. This was likely due to the different ecological requirements of these species, with the trees taking advantage of the persistence of both warming and decrease in snow cover duration, especially during the transition seasons (for example, spring and autumn), when the harsher climatic conditions (here represented by the air temperature trends in April and precipitation in November) may prevent the survival and growth of the young individuals. The role of transition seasons in explaining the biotic responses to climate change has been highlighted particularly in the Arctic (Lüers and others 2014; Cannone and others 2016) and would deserve more attention also for the alpine environment.
A slight relationship with land-use was detected over the whole study period (1875–2015), but with a strength that was less than half that of the relationship with climatic factors (Table 2). Notably, land-use was not related to the recruitment of tree species in the recent period (for example, since 1970) (Table 2). This trend could be explained considering the hypothesis that the relation between tree establishment and grazing intensity would not be linear and trees would not be able to establish above a threshold of grazing intensity. It is likely that since 1970s grazing intensity dropped below this hypothetical threshold and that the climate amelioration provided by warming allowed the observed pulse in tree recruitment. Indeed, since 1970s to nowadays, the amount and composition of livestock at the Alpe Braulio farm did not change, indicating that for the last 45 years, climate warming has been the main driver of tree colonization, together with a possible facilitation/limitation effects by shrub community.
Nurse Effect of Shrubs on Tree Recruitment
Many authors have pointed out how changes in the treeline ecotone could be explained by other factors rather than by climate alone (that is, Turnbull and others 2000; Graae and others 2011). In the stressful alpine ecosystem, interaction with shrubs, as well as with other vegetation types, may protect and facilitate tree germination (that is, Batllori and others 2009; Holmgren and others 2015), but it may also act as a constraint (that is, Frei and others 2018). In our case study, the oldest tree individuals were mainly associated with shrub-dominated areas and located at a distance no more than 2 m from the nearest individuals of R. ferrugineum (Figure 5). The frequency of tree species indicated a species-specific response to shrub co-occurrence as P. mugo indicated a higher preference than L. decidua for a closer relation of adult trees with shrubs (Figure 5). However, our data show that the relationship of trees with shrubs changed with the developmental stage of trees. In particular, seedlings of both tree species showed a larger preference for grassland communities, indicating no need for a nurse effect for their seed germination (Figure 5). A further confirmation of the lack of facilitation in the first developmental stages of trees is provided by the evidence that tree species were found up to 1 km away and up to 130 m (L. decidua) and 169 m (P. mugo) higher than any R. ferrugineum patches, indicating the ability of these species to germinate and survive without the co-occurrence of shrubs, probably in sheltered micro-sites (that is, Dullinger and others 2003; Batllori and others 2009).
Therefore, the presence of a “nurse effect” of shrubs was not confirmed from our results as a facilitation was observed only for adult tree individuals (Figure 5). The reasons for the low and species-specific response can be explained by (i) the ecological requirements of the tree species, (ii) the developmental stages of the shrub population and (iii) the competition and allelopathy of the shrubs.
Concerning the ecological niche (i), the seedlings of P. mugo show a weaker relation than seedlings of L. decidua with shrubs (Figure 5), in agreement with the higher frost resistance and lower humidity requirement of P. mugo (Table 1 Supplementary Materials; Ladinig and others 2015; Pignatti 2005). This finding, combined with the ability of shrubs to modify the snow, consequently reducing frost exposure and increasing soil moisture (that is, Sturm and others 2005), is one of the possible reasons why seedlings of P. mugo mainly spread in the more open areas. This is in agreement with results from the northern central Alps, where the recruitment of P. mugo was negatively affected by an extremely dense and tall vegetation and positively affected by ground disturbance, able to create gaps and safe micro-sites for seed germination (Dullinger and others 2003). However, it is notable that the relationship with shrubs changes when considering the saplings and, above all, the adults of both tree species. Similar trends were observed by Bullock and others (2009) in their study of pines invading heathland in Britain.
Overall, the facilitation effect may turn into inhibition (and vice versa) soon after tree germination or during particular phenological and developmental stages of the surrounding species and vegetation (Loranger and others 2017; Angulo and others 2019). In our case study, the lack of a clear nurse effect can be attributed to the ongoing process of shrub establishment and encroachment (ii). Indeed, since the 1970s, we reported a decreasing number of new R. ferrugineum individuals, a phenomenon that is comparable with the final stage of an invading process (Pornon and Doche, 1995; Pasche and others 2004). In this context, tree species started to germinate in the 1960s–1970s (P. mugo since 1960; L. decidua since 1972), with the older individuals found in shrub-dominated patches (despite not statistically significant). Moreover, in shrub patches, we reported the largest stem basal diameter, height and canopy diameter (p < .001) of tree species. The apparent discrepancy that we found between tree frequency and plant size within the shrub community lies in the coverage change of R. ferrugineum during the tree recruitment period (1960s–nowadays). Indeed, as suggested by Holmgren and others (2015), the increase in shrub cover depressed the emergence and survival of seedlings, but enhanced the condition and growth of the older trees, which had established before the complete canopy enclosure of R. ferrugineum. According to Holmgren and others (2012), the lack of the positive influence of shrub cover was particularly evident in the less shade-tolerant species (P. mugo).
Eventually, shrubs and other plant communities are able to affect the tree distribution also via chemical processes (iii). Soil nutrient status and the chemical properties, such as total P and total phenolic acids, are strongly related to ericaceous dominance, in particular in the organic horizon (Bloom and Mallik 2006), although Pornaro and others (2017) found that both pH and N content were not related to species coverage in a subalpine grassland. Other studies report how R. ferrugineum can better exploit soil nutrients than tree species (that is, Angulo and others 2019), leading to a lower reserve accumulation in L. decidua and P. mugo under a full shrub cover (Loranger and others 2017). Allelopathy may also occur (Angers-Blondin and others 2018), because of the presence of toxic diterpenes in the R. ferrugineum leaves, with a possible negative influence on cellular activity of trees (Louis and others 2010). However, further investigations are needed to validate this point.
Conclusions
Our data provided evidence of an increase in shrub and tree abundance along an alpine tundra ecotone related to air temperature and snow cover duration changes, in line with findings from other high-latitude and high-altitude environments, and provided one of the few case studies of this process in the European Alps. We found different temporal and spatial colonization patterns of shrub (R. ferrugineum) and tree (L. decidua, P. mugo) species. The former responded to the climate amelioration that occurred immediately after the Little Ice Age (LIA) and more recently was mainly affected by snow cover duration and spring temperature changes. Tree recruitment occurred only in the last decades of the twentieth century, sustained by a strong increase in air temperature (especially in spring), growing season lengthening and persistence of warming in the time periods after recruitment, allowing survival and growth of the new individuals. Land-use change did not exert any statistically significant influence on shrubs. It showed a weak relationship with trees, but only when considering the whole study period (1875–2015). Our data show that there was not a “nurse effect” of shrubs on trees, and that this kind of biotic interaction changed with the developmental stage of the involved species, requiring further investigations.
Future scenarios of climate change indicate a further and intense warming, and our data show that it is likely that shrub and tree encroachment will continue (likely at higher elevations), with relevant consequences on biodiversity, floristic composition, habitat types, food webs and ecosystem services of these extremely vulnerable and valuable high-elevation alpine ecosystems.
References
Angers-Blondin S, Myers-Smith IH, Boudreau S. 2018. Plant–plant interactions could limit recruitment and range expansion of tall shrubs into alpine and Arctic tundra. Polar Biology 41:2211–19.
Angulo MA, Ninot JM, Peñuelas J et al. 2019. Tree Sapling Responses to 10 Years of Experimental Manipulation of Temperature, Nutrient Availability, and Shrub Cover at the Pyrenean treeline. Frontiers in Plant Science . https://doi.org/10.3389/fpls.2018.01871.
Anthelme F, Villaret J-C, Brun J-J. 2007. Shrub encroachment in the Alps gives rise to the convergence of sub-alpine communities on a regional scale. Journal of Vegetation Science 18:355–62.
Auer I, Böhm R, Jurkovic A et al. 2007. HISTALP - historical instrumental climatoogical surface time series of the Greater Alpine Region. International Journal of Climatology 27:17–46.
Batllori E, Gutiérrez E. 2008. Regional tree line dynamics in response to global change in the Pyrenees. Journal of Ecology 96:1275–88.
Batllori E, Blanco-Moreno JM, Ninot JM et al. 2009. Vegetation patterns at the alpine treeline ecotone: the influence of tree cover on abrupt change in species composition of alpine communities. Journal of Vegetation Science 20:814–25.
Beniston M. 2012. Is snow in the Alps receding or disappearing? WIREs Climate Change . https://doi.org/10.1002/wcc.179.
Bernareggi G, Carbognani M, Petraglia A et al. 2015. Climate warming could increase seed longevity of alpine snowbed plants. Alpine Botany 12:69–78.
Bloom RG, Mallik AU. 2006. Relationships between ericaceous vegetation and soil nutrient status in a post-fire Kalmia angustifolia-black spruce chronosequence. Plant Soil 289:211–26.
Böhm R, Auer I, Brunetti M et al. 2001. Regional temperature variability in the European Alps: 1760-1998 from homogenized instrumental time series. International Journal of Climatology 21:1779–801.
Bonnrer FT, Karrfalt RP, Nisley RG 2008. The woody seed plant manual. USDA, Forest Service, Agricolture Handbook 727.
Boulanger-Lapointe N, Lévesque E, Boudreau S et al. 2014. Population structure and dynamics of Arctic willow (Salix arctica) in the High Arctic. Journal of Biogeography 41:1967–78.
Büntgen U, Hellmann L, Tegel W et al. 2015. Temperature-induced recruitment pulses of Arctic dwarf shrub communities. Journal of Ecoogy 103:489–501.
Caccianiga M, Compostella C. 2012. Growth forms and age estimation of treeline species. Trees 26:331–42.
Camarero JJ, Linares JC, Garcìa-Cervigo AI et al. 2017. Back to the Future: The Responses of Alpine Treelines to Climate Warming are constrained by the Current Ecotone Structure. Ecosystems 20:683–700.
Cannone N, Pignatti S. 2014. Ecological responses of plant species and communities to climate warming: upward shift or range filling processes? Climatic Change 123(2):201–14.
Cannone N., Guglielmin M., Hauck C, and others 2003. The impact of recent glacier fluctuation and human activities on permafrost distribution, Stelvio Pass (Italian Central-Eastern Alps). Proceedings of the 8th International Conference on Permafrost, Zurich (CH), 21–25 July 2003, Vol 1: 125–130.
Cannone N, Sgorbati S, Guglielmin M. 2007. Unexpected impacts of climate change on alpine vegetation. Frontiers in Ecology and the Environment 5(7):360–5.
Cannone N, Augusti A, Malfasi M et al. 2016. The interaction of biotic and abiotic factors at multiple spatial scales affects the variability of CO2 fluxes in polar environments. Polar Biology 39(9):1581–96. https://doi.org/10.1007/s00300-015-1883-9.
Dofour-Tremblay G, Lévesque E, Boudreau S. 2012. Dynamics at the treeline: differential responses of Picea mariana and Larix laricina to climate change in eastern subarctic Québec. Environmental Research Letters 7:044038.
Dullinger S, Dirnböck T, Grabherr G. 2003. Patterns of shrub invasion into high mountain grasslands of the northern calcareous Alps, Austria. Arctic, Antarctic, and Alpine Research 35:434–41.
Elmendorf SC, Henry GHR, Hollister RD et al. 2012a. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecology Letters 15:164–75.
Elmendorf SC, Henry GHR, Hollister RD et al. 2012b. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Climate Change 2:453–7.
Fernàndez-Martínez J, Fransi MA, Fleck I. 2016. Ecophysiological responses of Betula pendula, Pinus uncinata and Rhododendron ferrugineum in the Catalan Pyrenees to low summer rainfall. Tree Physiology 36:1520–35.
Francon L, Corona C, Roussel E et al. 2017. Warm summers and moderate winter precipitation boost Rhododendron ferrugineum L. growth in the Taillefer massif (French Alps). Science of the Total Environment 586:1020–31.
Frei ER, Bianchi E, Bernareggi G et al. 2018. Biotic and abiotic drivers of tree seedling recruitment across an alpine treeline ecotone. Scientific Reports 8:1094. https://doi.org/10.1038/s41598-018-28808-w.
Frost GV, Epstein HE. 2014. Tall shrub and tree expansion in Siberian tundra ecotones since the 1960s. Global Change Biology 20:1264–77.
Gamache I, Payette S. 2005. Latitudinal response of subarctic tree lines to recent climate change in eastern Canada. Journal of Biogeography 32:849–62.
Gehrig-Fasel J, Guisan A, Zimmermann NE. 2007. Tree line shifts in the Swiss Alps: climate change or land abandonment? Journal of Vegetation Science 18:571–82.
Giaccone E, Colombo N, Acquaotta F et al. 2015. Climate variations in a high altitude Alpine basin and their effects on a glacial environment (Italian Western Alps). Atmosfera 28:117–28.
Giacomini V, Pignatti S. 1955. Flora e vegetazione dell’Alta Valle del Braulio con speciale riferimento ai pascoli di altitudine. Memorie della Società Italiana di Scienze Naturali 11:47–238.
Gottfried M, Pauli H, Futschik A et al. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2:111–5.
Graae BJ, Ejrnaes R, Lang SI et al. 2011. Strong microsite control of seedling recruitment in tundra. Oecologia 166:565–76.
Grau O, Ninot JM, Cornelissen JHH et al. 2013. Similar tree seedling responses to shrubs and to simulated environmental changes at Pyrenean and subarctic treelines. Plant Ecology and Diversity 6:329–42.
Guglielmin M, Siletto GB 2000. Carta della Criosfera. Regione Lombardia, Direzione Generale Territorio ed Edilizia Residenziale
Guglielmin M, Donatelli M, Semolice M et al. 2018. Ground surface temperature reconstruction for the last 500 years obtained from permafrost temperatures observed in the Stelvio ShareCE1 Borehole, Italian Alps. Climate of the Past 14:1–16.
Holmgren M, Gomez-Aparicio L, Quero JL et al. 2012. Non-linear effects of drought under shade: reconciling physiological and ecological models in plant communities. Oecologia 169:293–305.
Holmgren M, Lin C-Y, Murillo JE et al. 2015. Positive shrub–tree interactions facilitate woody encroachment in boreal peatlands. Journal of Ecology 103:58–66.
Holtmeier FK, Broll G. 2017. Treelines-Approaches at Different Scales. Sustainability 9:808. https://doi.org/10.3390/su9050808.
IPCC Working Group II. 2014. Climate change 2014: Impacts, adaptation, and vulnerability. Geneva, Switzerland: IPCC.
Komac B, Kefi S, Nuche P et al. 2013. Modeling shrub encroachment in subalpine grasslands under different environmental and management scenarios. Journal of Environmental Management 121:160–9.
Körner C, Paulsen J. 2004. A world-wode study of high altitude treeline temperatures. Journal of Biogeography 31:713–32.
Körner C. 2003. Alpine plant life: functional plant ecology of high mountain ecosystems; with 47 tables. Berlin: Springer.
Körner C. 2012. Alpine treelines. Springer, Basel: Functional ecology of the global high elevation tree limits.
Ladinig U, Pramsohler M, Bauer I et al. 2015. Is sexual reproduction of high-mountain plants endangered by heat? Oecologia 177:1195–210.
Lenoir J, Gégout JC, Marquet PA et al. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–71.
Leonelli G, Masseroli A, Pelfini M. 2016. The influence of topographic variables on treeline trees under different environmental conditions. Physical Geography 37:56–72.
Loranger H, Zotz G, Bader MY. 2017. Competitor or facilitator? The ambiguous role of alpine grassland for the early establishment of tree seedlings at treeline. Oikos 126:1625–36.
Louis A, Lechtenbergm M, Deters A et al. 2010. Phytochemical characterization of Rhododendron ferrugineum and in vitro assessment of an aqueous extract on cell toxicity. Planta Medicine 76:1550–7.
Lüers J, Westermann S, Piel K, Boike J. 2014. Annual CO2 budget and seasonal CO2 exchange signals at a high Arctic permafrost site on Spitsbergen, Svalbard archipelago. Biogeosciences 11:6307–22.
Mayr S, Beikircher B, Obkircher M-A. 2010. Hydraulic plasticity and limitations of alpine Rhododendron species. Physiological Ecology 164:321–30.
Myers-Smith IH, Hik DS. 2018. Climate warming as a driver of tundra shrubline advance. Journal of Ecology 106:547–60.
Myers-Smith IH, Hallinger M, Blok D et al. 2015. Methods for measuring arctic and alpine shrub growth: a review. Earth-Science Reviews 140:1–13.
Neuner G. 2014. Frost resistance in alpine woody plants. Frontiers in Plant Science 5:1–13.
Pasche F, Armand M, Gouaux P et al. 2004. Are meadows with high ecological and patrimonial value endangered by heathland invasion in the French central Pyrenees? Biological Conservation 118:101–8.
Pignatti S. 1982. Flora d’Italia. Edagricole, Bologna: Three volumes.
Pignatti S. 2005. Bioindicator values of vascular plants of the Flora of Italy. Braun-Blanquetia 39:1–97.
Pornaro C, Schneider MK, Leinauer B et al. 2017. Above- and belowground patterns in a subalpine grassland-shrub mosaic. Plant Biosystems 151:493–503.
Pornon A, Doche B. 1995. Age structure and dynamics of Rhododendron ferrugineum L. populations in the Northwestern French Alps. Journal of Vegetation Science 7:265–72.
Pornon A, Escaravage N, Till-Bottraud I et al. 1997. Variation of reproductive traits in Rhododendron ferrugineum L. (Ericaceae) populations along a successional gradient. Plant Ecology 130:1–11.
Scherrer SC, Wüthrich C, Croci-Maspoli M et al. 2013. Snow variability in the Swiss Alps 1864–2009. International Journal of Climatology 33:3162–73.
Schmidt NM, Baittinger C, Forchhammer MC. 2006. Reconstructing century-long snow regimes using estimates of high arctic Salix arctica radial growth. Arctic, Antarctic and Alpine Research 38:257–62.
Sturm M, Douglas T, Racine C et al. 2005. Changing snow and shrub conditions affect albedo with global implications. Journal of Geophysical Research . https://doi.org/10.1029/2005JG000013.
Turnbull LA, Crawley MJ, Kees M. 2000. Are plant populations seed-limited? A review of seed sowing experiment. Oikos 88:225–38.
Vittoz P, Engler R. 2007. Seed dispersal distances: a typology based on dispersal modes and plant traits. Botanica Helvetica 117:109–24.
Walther G-R. 2003. Plants in a warmer world. Perspectives in Plant Ecology, Evolution and Systematics 6:169–85.
Acknowledgements
We thank Stelvio National Park for logistical support and a2a for providing the climatic data. We thank the former Rector of Insubria University Prof. Renzo Dionigi for funding the Insubria Dendrochronological laboratory. This article contributes to the Project PRIN (Projects of Relevant National Interest) RESACC “Responses of Sensitive Alpine Ecosystems to Climate Change” (2015N8F555).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Contributions
FM performed the field activity, analysed the data and wrote the paper. NC conceived and designed the study, participated in field activity, analysed the data and wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malfasi, F., Cannone, N. Climate Warming Persistence Triggered Tree Ingression After Shrub Encroachment in a High Alpine Tundra. Ecosystems 23, 1657–1675 (2020). https://doi.org/10.1007/s10021-020-00495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-020-00495-7