Abstract
Background
Poly-brominated diphenyl ethers (PBDEs) and heavy metals are persistent pollutants in Yangtze River basin, China and also around the globe. In the exposure reality, they may have sequential exposures and long-term outcomes. Yet, the effects combining these two aspects remained largely unexplored. At present, the effects of 2,2′,4,4′-tetra-brominated diphenyl ether (BDE47) and lead (Pb) on Caenorhabditis elegans were studied with two sequential arrangements. One was first exposure to BDE47 and then to Pb (or vice versa) in one generation, and the other one was an early exposure to BDE47 in the parent generation (F0) and then a later exposure to Pb in the offspring (F1) (or vice versa).
Results
On growth, sequential Pb-BDE47 exposure caused inhibition in exposure but stimulation in recovery, showing similarity to individual Pb results. Meanwhile, the opposite sequential BDE47-Pb exposure showed inhibition in both exposure and recovery, similar to those of individual BDE47 results. On behavior, the effects of sequential exposure were significantly different from individual results without any similarity. In transgenerational effects, F0 Pb exposure with F1 BDE47 exposure (Pb-F1-BDE47) inhibited growth, similar to the transgenerational results of individual Pb exposure. Meanwhile, the recovery effects were similar to the transgenerational results of individual BDE47. At the same time, Pb-F1-BDE47 exposure significantly hindered the recovery of behavior while the opposite BDE47-F1-Pb exposure resulted in different results. The behavioral effects in F1 showed negative correlation with the contents of γ-aminobutyric acid (GABA), while those in F1 were positively correlated with the activities of acetylcholine esterase (AChE).
Conclusions
Sequential exposure to BDE47 and Pb within one generation or over generations showed significant different effects from individual results. Further studies are still needed to investigate the toxicity patterns and underlying mechanisms.
Similar content being viewed by others
Background
Poly-brominated diphenyl ethers (PBDEs) and heavy metals are persistent pollutants in Yangtze River basin, China and also in other countries around the globe. They were found in soil and dust [1,2,3,4], and also in biota (e.g., fish) [5, 6], and even in human tissues [7]. They can provoke various hazards, e.g., their well-known neuro-developmental toxicities [8,9,10]. Therefore, it is important to fully demonstrate their hazard to environmental organisms and human health, especially in the complex exposure reality.
Notably, the presence of PBDEs was accompanied with heavy metals (e.g., lead) in e-waste recycling areas [11]. Yet, there are only limited studies on their combined toxicities. For example, the co-exposure of PBDEs and Pb disrupted reproduction and thyroid endocrine function in zebrafish adults with subsequent influences on the hormones in the eggs [12]. Moreover, effects of the co-exposure on the offspring showed different reproductive outcomes from individual exposure and resulted in transgenerational neurobehavioral deficits in the offspring [12]. Yet, the exposure reality is more complicated than such co-exposure effects.
In the aspect of combined toxicities, the effects of sequential exposure should be considered due to the fact that pollutants have sequential occurrences out of geographical influences, accidental contamination and application/emission histories. It was found that the presence of Pb promoted the PBDEs bioaccumulation in earthworms over time [13], while the presence of PBDEs reduced the Pb accumulation [14]. These studies led to an assumption that sequential exposure to PBDEs and Pb can result in different toxicities, which is essential in assessing their actual ecological outcomes.
Regarding the persistence, PBDE and Pb showed long-term influences over generations. Perinatal exposure to 2,2′,4,4′-tetra-brominated diphenyl ether (BDE47) significantly damaged the normal spermatogenesis of adult rats [15, 16]. Lead (Pb) inhibited the growth of daughters (F1) of Caenorhabditis elegans more than that of parents (F0) [17]. Interestingly, in a transgenerational study on marine invertebrate (Crepidula onyx), parental exposure to BDE47 caused developmental toxicities on the offspring, but a continuous exposure in the offspring reduced the toxicities, causing potential adaptation/acclimation responses [18]. Therefore, sequential exposure was expected to influence the transgenerational effects.
So far, seldom studies combined both sequential and transgenerational exposure to illustrate the hazards of pollutants in the exposure reality. This situation was mainly resulted from the tremendous experimental efforts. Fortunately, it can be facilitated by C. elegans, a free-living nematode. This nematode has significant ecological function by connecting lower and higher trophic levels in food webs. Its multiple indicators, especially those related with the neuro-developmental toxicities of PBDEs and Pb, had been employed to demonstrate both combined [19, 20] and transgenerational effects of pollutants [17, 21].
In the present study, the effects of BDE47 and Pb were studied on C. elegans with considerations on sequential and transgenerational exposure. One exposure arrangement was first exposure to BDE47 and then to Pb (or vice versa) in one generation, and the other one was an early exposure to BDE47 in the parent generation (F0) and then a later exposure to Pb in the offspring (F1) (or vice versa). Growth, behavior and the contents of γ-aminobutyric acid (GABA) and activities of acetylcholine esterase (AChE) were measured. Out findings demonstrated that sequential exposure effects were indeed different from individual ones. Such effects were also observed in recovery potentials and transgenerational outcomes. Further studies are needed to reveal the toxicity patterns and the underlying mechanisms.
Materials and methods
Tested chemicals
The target PBDE was 2,2′,4,4′-tetra-brominated diphenyl ethers (BDE47, CAS-RN: 5436-43-1, Accustandrad, Inc., USA). The chosen heavy metal was Pb in the form of Pb(NO3)2 (CAS-RN: 10099-74-8, Sigma Aldrich). The stock solution of BDE47 (100 mg/L) was prepared with sterile K-medium containing 1% dimethyl sulfoxide (DMSO, Amresco, USA). The stock solution of Pb (10 mg/L) was prepared with sterile K-medium. The stock solutions would be diluted by 1% DMSO in sterilized K-medium in exposure. The actual exposure concentrations were 10 μg/L for Pb [3] and 0.1, 1, 10 and 100 μg/L for BDE47 [1, 11] regarding their environmental realistic levels.
Preparation of nematode
Caenorhabditis elegans (wild-type N2) and its food E. coli OP50 were cultured according to previous studies [22, 23]. In brief, E. coli OP50 was cultured in sterile lysogeny broth (LB) culture medium, and C. elegans was cultured on solid nematode growth medium (NGM) plates. Gravid nematodes were bleached for 5–10 min at room temperature in fresh clorox solutions containing 0.5 M NaOH and 1% NaOCl (diluted from antiformin, 4–6%, Sinopharm Group Co. Ltd., China) [23]. Then, the age-synchronized eggs were further cultured on NGM agars with bacterial lawn for 36 h at 20 °C to obtain L3 nematodes [17, 21]. Before used, L3 nematodes were washed in sterile K-medium before their use in experiments.
Experiment design
The toxicity experiments were performed according to previous study with necessary modifications [24]. The aqueous exposure was performed in 24-well sterile plates (with cover, Corning, Inc., USA). The overall experiment had 10 plates in total, including two plates for control groups (one with sterilized K-medium, and another with 1% DMSO in sterilized K-medium), four plates for four BDE47 concentrations (0.1, 1, 10 and 100 μg/L), and four plates for one Pb concentration (10 μg/L). All 24 wells in each plate had the same arrangement so as to ensure enough nematodes for following experiments. Every well in the plate contained 500 μL toxicant or control solution, 300 μL K-medium containing bacteria suspensions and 200 μL sterile K-medium L3 C. elegans. Sterilized water (500 μL) was added in the interspaces between wells to keep the humidity and decrease the water evaporation during the exposure period.
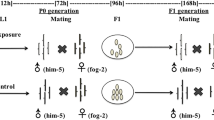
The sequential exposure arrangements are shown in Fig. 1. In each group, there were 8 arrangements in total. They were briefly explained as follows. (1) In the first arrangement, nematodes experienced 24-h exposure to one chemical and then were collected, washed with sterile water and transferred on to NGM agar for growth and behavior test or into centrifuge tubes for biochemical analysis. (2) In the second arrangement, nematodes experienced 12-h exposure to one chemical. Next, they were collected, washed and experienced another 12-h exposure to another chemical. Then, they were collected, washed and used to measure effects. Notably, nematodes from different BDE47 concentrations experienced Pb exposure at the same concentration, while those from Pb exposure were separated to experience BDE47 exposure with four concentrations. (3) In the third arrangement, nematodes experienced 24-h exposure. Next, they were collected, washed and transferred into sterile K-medium for 24 h recovery. Then, they were collected, washed and used to measure effects. (4) In the fourth arrangement, nematodes experienced 12-h exposure to one chemical, 12-h sequential exposure to another chemical, and also 24-h recovery before they were used to measure effects. (5–8) In the remaining 4 arrangements, nematodes experienced 24-h exposure, 24-h recovery, and then they were synchronized to obtain the eggs (F1). Next, the eggs grew to L3 nematodes on NGM agar with bacterial lawn for 36 h at 20 °C. Then, the F1 nematodes experienced 24-h exposure to the same or another chemical with or without 24-h recovery before they were used to measure effects. Each arrangement had its own concurrent control nematodes that experienced the same procedure as those experienced exposure.
Growth and behavior indicators
Growth and behavior indicators were measured according to earlier studies [21]. In brief, the nematodes were allowed to crawl on NGM agar without bacterial lawn for 2 h. Such a crawling would allow the evaporation of extra water and help distribution of nematodes from the crowded pile. Then, the nematode images were taken by a dissecting microscope with a computer. The nematode growth in the form of body length (BL) was indicated by half the length of a polyline following the midline from the nematode head to the tail and back to the head. At least 20 replicates were measured for the BL in each treatment. Next, the nematode moving videos were captured with an interval of 60 s. Then, body bending frequency (BBF) was counted as the times when the direction of posterior pharyngeal bulb changed along the vertical direction of the traveling path. At least 6 animals were measured for replication.
Biochemical assay
The amounts of γ-aminobutyric acid (GABA) and activities of acetylcholine esterase (AChE) were measured by Shanghai Ketao Biotechnology, Ltd. In brief, the nematodes were washed with the buffer of phosphate-buffered saline (PBS, pH 7.0). After a centrifugation at 10,000 rpm for 5 min at 4 °C, the supernatants were discarded and the pellets were stored at − 80 °C until assayed. The pellets were ground by grinding rods with ice bath. Then, 200 ice-cold PBS were pelleted onto the grinding rods to wash the solutions back to the centrifuge tubes. After centrifugation, the supernatants were used to determine the contents of GABA and the activities of AChE with the commercial ELISA kits (R&D Systems, Inc., USA). The amounts of the total protein (TP) in each sample were also measured. All biochemical indices were expressed as their proportions against the TP amounts.
Data presentation and statistical analysis
All indicators were calculated as the percentages of the average value of the control (POC) [25]. Through this way, the values in the control were all normalized to 100%. POC values lower than 100% demonstrated inhibitory effects while those higher than 100% demonstrated stimulatory ones. In figures, the data were expressed as mean ± standard error. The POC values among groups were analyzed by two-way ANOVA (using Tukey as post hoc test), and the probability levels of 0.05 were considered statistically significant (p < 0.05). The correlations between indices were analyzed with Spearman rank correlation through Origin 9.0 (USA) [26]. Notably, the results in the absolute control (K-medium) and the solvent control (1% DMSO in K-medium) were not significantly different from each other. Therefore, the average values of both controls were used to demonstrate the control values.
Results
Effects of Pb or BDE47, and their sequential exposure on the nematode growth
The effects of individual exposure to Pb or BDE47 on the nematode growth are shown in Fig. 2a. In BDE47 results, the POC values were 93.1%, 86.0%, 81.6% and 80.6% in exposure at 0.1, 1, 10 and 100 μg/L, respectively. The POC values were lower than the control (p < 0.05), indicating that BDE47 inhibited the body length. The POC values ranged from 91.0 to 93.6% in recovery at four concentrations. The POC values were lower than the control (p < 0.05), but higher than those in exposure results (p < 0.05). Such comparison results indicated that BDE47 still inhibited the growth with limited recoveries. The Pb results showed that it inhibited the growth in exposure with a POC of 90.9% (p < 0.05) but stimulated it in recovery with a POC of 103.9% (p < 0.05), showing significant recoveries.
The effects of individual Pb or BDE-47 (a) and their sequential exposure (b) on the body length of Caenorhabditis elegans. The sequential exposure was a 12-h exposure to BDE47 and another 12-h exposure to Pb (or vice versa). Blank column: exposure effects; shadow column: recovery effects. The average body length was 1.13 mm in the control nematodes. *Significantly different from the control, p < 0.05. Data represent the means and standard error
The effects of sequential exposure to Pb and BDE47 on the nematode growth are shown in Fig. 2b. Results showed that sequential exposure to Pb and BDE47 (Pb-BDE47) inhibited the body length in exposure with POC values ranging from 89.5 to 94.0%, while they stimulated it in recovery with POC values from 103.6 to 104.6%. The inhibition in exposure and stimulation in recovery were similar to the individual Pb results. Meanwhile, the sequential exposure to BDE47 and Pb (BDE47-Pb) inhibited the body length in both exposure and recovery, with POC values ranging from 90.4 to 97.5%. The inhibition in both exposure and recovery was similar to the individual BDE47 results. Yet, the inhibition by BDE47-Pb was less than individual BDE47 exposure. The results demonstrated that the exposure experience at earlier life stage (i.e., the first 12-h exposure) defined the toxicity types on the growth in the end. The sequential exposure can result in different toxicity levels, indicating potential toxicity interactions.
Effects of Pb or BDE47, and their sequential exposure on the nematode behavior
The effects of individual exposure to Pb or BDE47 on the nematode body bending frequency (BBF) are shown in Fig. 3a. In BDE47 results, it inhibited BBF and the inhibition showed a concentration dependence where the POC was as low as 56.6% (p < 0.05) at 100 μg/L. In the recovery, BDE47 stimulated BBF with POC values ranging from 121.7 to 128.5% (p < 0.05). In Pb results, it significantly stimulated BBF with a POC of 144.4% (p < 0.05) in exposure, while inhibited it with a POC of 73.7% (p < 0.05) in recovery. Notably, effects of BDE47 and Pb showed opposite changes from inhibition (or stimulation) in exposure to stimulation (or inhibition) in recovery.
The effects of individual Pb or BDE-47 (a) and their sequential exposure (b) on the body bending frequency (BBF) of Caenorhabditis elegans. The sequential exposure was a 12-h exposure to BDE47 and another 12-h exposure to Pb (or vice versa). Blank column: exposure effects; shadow column: recovery effects. The average BBF was 6 bends/10 s in the control nematodes. *Significantly different from the control, p < 0.05. Data represent the means and standard error
The effects of sequential exposure to Pb and BDE47 on the nematode BBF are shown in Fig. 3b. In Pb-BDE47 results, they slightly inhibited the BBF in exposure. In recovery, they stimulated BBF with POC value as high as 147.3% (p < 0.05) at Pb 10 μg/L and BDE47 0.1 μg/L, while they inhibited BBF at other BDE47 concentrations. In BDE47-Pb results, they showed inhibition on BBF with POC values ranging from 54.1 to 78.4% (p < 0.05). In recovery, the inhibition was greatly recovered with POC values from 84.6 to 115.4% (p < 0.05). The effects of sequential exposure to Pb and BDE47 did not show clear similarity to the individual Pb or BDE47 results.
The effects of individual exposure to Pb or BDE47 and their sequential exposure on the AChE and GABA are shown in Additional file 1: Figure S1. The correlations between effects on BBF and those on neuro-transmitters were analyzed via Spearman rank correlations, and the results are listed in Table 1. The results showed that the effects of Pb or BDE47 and their sequential exposure on BBF were negatively correlated with those on GABA. Such results indicated that effects on behavior in F0 mainly involved GABA regulation.
Transgenerational effects of Pb, BDE47 and their sequential exposure on growth
The effects of individual exposure to Pb or BDE47 on the nematode growth in the offspring generation (F1) are shown in Fig. 4a. Results showed that individual exposure to BDE47 slightly inhibited F1 growth (p < 0.05). The inhibition was partially recovered to normal level and even changed into slight stimulatory effects (p < 0.05). The effects of BDE47 on F1 were significantly lower than those on F0 (Fig. 2a). Meanwhile, individual exposure to Pb significantly inhibited F1 growth with non-significant recoveries. The toxicities of Pb on F1 were significantly worse than those within one generation (Fig. 2a).
The effects of individual Pb or BDE-47 (a) and their sequential exposure (b) on the body length of Caenorhabditis elegans offspring (F1). The sequential exposure was an early exposure to BDE47 in the parent and a later exposure to Pb in the offspring (or vice versa). Blank column: exposure effects; shadow column: recovery effects. The average body length was 1.13 mm in the control nematodes. *Significantly different from the control, p < 0.05. Data represent the means and standard error
The effects of sequential exposure to Pb and BDE47 on F1 growth are shown in Fig. 4b. The results showed that 10 μg/L Pb exposure in F0 with BDE47 exposure in F1 (i.e., Pb-F1-BDE47) greatly inhibited F1 growth with significant recoveries which even showed stimulatory effects. Meanwhile, the sequential exposure to BDE47 in F0 and Pb in F1 (i.e., BDE47-F1-Pb) resulted in slight inhibition on F1 growth with slight recoveries. The results also demonstrated that early exposure to Pb in F0 resulted in significant inhibition on growth in F1, which was similar to the transgenerational effects of individual Pb exposure. Meanwhile, later exposure to BDE47 in F1 significantly influenced the recovery effects which were similar to the transgenerational effects of individual BDE47 exposure.
Transgenerational effects of Pb, BDE47 and their sequential exposure on behavior
The effects of individual exposure to Pb or BDE47 on the nematode BBF in F1 are shown in Fig. 5a. Results showed that BDE47 significantly inhibited F1 BBF in exposure with significant recoveries at the lowest concentration. Both inhibitory and recovery effects in F1 were lower than those within one generation. In Pb results, it did not inhibit the BBF in F1 without significant recoveries. Such results were different from the stimulation in exposure and inhibition in recovery within one generation (Fig. 3a). That is to say, transgenerational effects of individual BDE47 or Pb were different from those within one generation.
The effects of individual Pb or BDE-47 (a) and their sequential exposure (b) on the body bending frequency (BBF) of Caenorhabditis elegans offspring (F1). The sequential exposure was an early exposure to BDE47 in the parent and a later exposure to Pb in the offspring (or vice versa). Blank column: exposure effects; shadow column: recovery effects. The average BBF was 6 bends/10 s in the control nematodes. *Significantly different from the control, p < 0.05. Data represent the means and standard error
The effects of sequential exposure to Pb and BDE47 on F1 BBF are shown in Fig. 5b. The Pb-F1-BDE47 exposure significantly inhibited BBF with non-significant recoveries. The negative effects of Pb-F1-BDE47 exposure were more severe than those of individual Pb effects over generations. Meanwhile, BDE47-F1-Pb exposure showed inhibition on F1 BBF with significant recoveries that even showed stimulatory effects. The significant recovery effects were also observed in the transgenerational effects of individual BDE47 (Fig. 5a). The overall results demonstrated that Pb exposure in F0 significantly hindered the recovery potentials in F1 while BDE47 exposure in F0 dramatically determined the recovery in F1.
The effects of individual exposure to Pb or BDE47 and their sequential exposure on the AChE and GABA in F1 are shown in Additional file 1: Figure S2. The Spearman rank correlation analysis results (Table 1) showed that the effects of Pb or BDE47 and their sequential exposure on F1 BBF were positively correlated with those on AChE. Such results indicated that effects on behavior in F1 primarily involved AChE regulation, instead of GABA in F0. Such results indicated that GABA and AChE functioned via different underlying mechanisms.
Discussion
Different effects between individual and sequential exposure
Effects of Pb and/or BDE47 on BL and BBF demonstrated that the exposure sequence indeed influenced the nematode responses and the exposure experience at earlier life stage can define the outcomes in the end. The influence of exposure sequence was also observed in earthworms that experienced different sequential exposure to BDE-209 and Pb [14, 27]. Such findings supported the DOHaD theory, i.e., the early experience to environmental stresses was the origin of health and disease in adults [28].
Various studies have demonstrated that pretreatment of chemicals or stressors can result in protective effects against the later exposure to other chemical. For example, pretreatment of selenium and antioxidants rescued the behavioral impacts and neurotoxic effects of Pb [29, 30]. Yet, the present study did not show such protective effects of earlier BDE47 or Pb exposure. Such differences might be resulted from the different modes of actions (MOAs) of BDE47 and Pb. For example, Pb exposure resulted in direct neurotoxicity via disturbances on excitatory and inhibitory synaptic transmission [31], while BDE47 might provoke indirect neurotoxicity after its disruption on hormone function [32]. Therefore, earlier exposure to Pb or BDE47 caused different intermediate state for the damage of the later exposure. Yet, further studies are needed to reveal potential patterns in the sequential exposure toxicities and the underlying mechanisms.
Transgenerational effects between individual and sequential exposure
Effects over generations collectively demonstrated that early exposure in parent significantly influenced the offspring responses. Such results supported the expansion of the DOHaD theory from embryo–adult to adult–embryo–offspring [33]. The transgenerational effects in the present study were also found in earlier studies. For example, transgenerational exposure to Pb induced its lethality on the offspring of D. magna [34]. The parental co-exposure to PBDEs and Pb enhanced developmental neurotoxicity in offspring of zebrafish [12]. The transgenerational studies urged special attentions on the long-term impacts of environmental pollutants.
Interestingly, there were different transgenerational effects between individual and sequential exposure. That is to say, the effects in the current generation were not a simple sum of experiences in the earlier and the current generation. There might be toxicity interaction underlying the effects over generations. Such toxicity interaction was implied in an earlier study where Pb exposure in earlier generations lowered the sensitivity of D. magna to mancozeb in later generations [35]. Notably, Pb and BDE47 can influence epigenetic regulations over generations. For example, Pb exposure altered DNA methylation in both the present generation [36] and the offspring [37]. The PBDE exposure formed DNA adducts [38] and disturbed the microRNA profiling [39] in the present generation and altered the DNA methylation and histone–protamine exchange in offspring adults [15, 16]. The epigenetic changes underlying the transgenerational effects might also contribute to the interactions.
Different mechanisms underlying effects on behavior over generations
The results also demonstrated that effects on behavior were negatively related with GABA but positively related with AChE. On the one hand, GABA showed both inhibitory responses (i.e., decreases in the transport of GABA and expression of its regulating genes [40]) and stimulatory ones (e.g., in zebrafish embryo [41]) in Pb neurotoxicity. Therefore, the negative correlation between GABA and behavioral responses in the present study did not reveal any particular pattern. On the other hand, the positive correlation between AChE and behavioral responses was widely reported. For example, AChE inhibition by Pb was accompanied with reduction in movement of bivalves [42] and AChE stimulation by sulfamethoxazole antibiotic was accompanied with increases in behavioral indicators [26]. Therefore, AChE might be a better choice to indicate the changes of neuro-transmitter underlying behavioral changes by pollutants. Notably, the correlation of behavioral responses with GABA in one generation exposure and that with AChE in transgenerational exposure suggested that GABA showed faster responses than AChE, while AChE had more persistent regulations over generations. The exact reason still needs future studies.
Conclusion
Effects of sequential exposure to BDE47 and Pb on growth and behavior were studied on C. elegans. Results demonstrated that effects of sequential exposure on growth within one generation or over generations relied on the chemical with an early appearance. Meanwhile, the sequential exposure caused different effects on behavior from individual results without any particular pattern. Moreover, the behavioral effects were negatively correlated with GABA in F0 but positively correlated with AChE in F1. Further studies are still needed to reveal the patterns in sequential exposure effects and the underlying mechanisms.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and supporting materials.
Abbreviations
- AChE:
-
Acetylcholine esterase
- BBF:
-
Body bending frequency
- BDE47:
-
2,2′,4,4′-Tetra-brominated diphenyl ether
- BL:
-
Body length
- DMSO:
-
Dimethyl sulfoxide
- GABA:
-
γ-Aminobutyric acid
- LB:
-
Lysogeny broth
- NGM:
-
Nematode growth medium
- Pb:
-
Lead
- PBDEs:
-
Poly-brominated diphenyl ethers
- PBS:
-
Phosphate-buffered saline
- POC:
-
Percentages of the average value of the control
- TP:
-
Total protein
References
Xu J, Qian W, Li J, Zhang X, He J, Kong D (2019) Polybrominated diphenyl ethers (PBDEs) in soil and dust from plastic production and surrounding areas in eastern of China. Environ Geochem Health 41:2315–2327
Cai Z, Jiang G (2006) Determination of polybrominated diphenyl ethers in soil from e-wast recycling site. Talanta 70:88–90
Jiang Y, Chao S, Liu J, Yang Y, Chen Y, Zhang A, Cao H (2017) Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 168:1658–1668
Wen B, Li L, Duan Y, Zhang Y, Shen J, Xia M, Wang Y, Fang W, Zhu X (2018) Zn, Ni, Mn, Cr, Pb and Cu in soil-tea ecosystem: the concentrations, spatial relationship and potential control. Chemosphere 204:92–100
Gandhi N, Gewurtz SB, Drouillard KG, Kolic T, MacPherson K, Reiner EJ, Bhavsar SP (2017) Polybrominated diphenyl ethers (PBDEs) in Great Lakes fish: levels, patterns, trends and implications for human exposure. Sci Total Environ 576:907–916
Brown TM, Lord SI, Schindler DW, Elliott JE (2018) Organohalogen contaminants in common loons (Gavia immer) breeding in Western Alberta, Canada. Chemosphere 202:438–445
Lu X, Xu X, Zhang Y, Zhang Y, Wang C, Huo X (2018) Elevated inflammatory Lp-PLA2 and IL-6 link e-waste Pb toxicity to cardiovascular risk factors in preschool children. Environ Pollut 234:601–609
Zhang B, Xu T, Huang G, Yin DQ, Zhang Q, Yang X (2018) Neurobehavioral effects of two metabolites of BDE-47 (6-OH-BDE-47 and 6-MeO-BDE-47) on zebrafish larvae. Chemosphere 200:30–35
Glazer L, Wells CN, Drastal M, Odamah KA, Galat RE, Behl M, Levin ED (2018) Developmental exposure to low concentrations of two brominated flame retardants, BDE-47 and BDE-99, causes life-long behavioral alterations in zebrafish. Neurotoxicology 66:221–232
Rahman A, Khan KM, Rao MS (2018) Exposure to low level of lead during preweaning period increases metallothionein-3 expression and dysregulates divalent cation levels in the brain of young rats. Neurotoxicology 65:135–143
Zhu X, Beiyuan J, Lau AY, Chen SS, Tsang DC, Graham NJ, Lin D, Sun J, Pan Y, Yang X, Li XD (2018) Sorption, mobility, and bioavailability of PBDEs in the agricultural soils: Roles of co-existing metals, dissolved organic matter, and fertilizers. Sci Total Environ 619:1153–1162
Chen L, Wang X, Zhang X, Lam PKS, Guo Y, Lam JCW, Zhou B (2017) Transgenerational endocrine disruption and neurotoxicity in zebrafish larvae after parental exposure to binary mixtures of decabromodiphenyl ether (BDE-209) and lead. Environ Pollut 230:96–106
Zhang W, Li JLJ, Lin K, Fu R (2016) Diverse impacts of a step and repeated BDE209-Pb exposures on accumulation and metabolism of BDE209 in earthworms. Chemosphere 159:235–243
Zhang W, Chen L, Liu K, Chen L, Lin K, Guo J, Liu L, Cui C, Yan Z (2014) Lead accumulations and toxic effects in earthworms (Eisenia fetida) in the presence of decabromodiphenyl ether. Environ Sci Pollut Res Int 21:3484–3490
Khalil A, Parker M, Brown SE, Cevik SE, Guo LW, Jensen J, Olmsted A, Portman D, Wu H, Suvorov A (2017) Perinatal exposure to 2,2′,4′4′-Tetrabromodiphenyl ether induces testicular toxicity in adult rats. Toxicology 389:21–30
Suvorov A, Shershebnev A, Wu H, Medvedeva Y, Sergeyev O, Pilsner JR (2018) Perinatal exposure to low dose 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) alters sperm DNA methylation in adult rats. Reprod Toxicol 75:136–143
Yu ZY, Chen XX, Zhang J, Wang R, Yin DQ (2013) Transgenerational effects of heavy metals on L3 larva of Caenorhabditis elegans with greater behavior and growth inhibitions in the progeny. Ecotoxicol Environ Safe 88C:178–184
Po BHK, Chiu JMY (2018) Transgenerational impairments of reproduction and development of the marine invertebrate Crepidula onyx resulted from long-term dietary exposure of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Environ Pollut 235:730738
Yu Z, Yin D, Deng H (2015) The combinational effects between sulfonamides and metals on nematode Caenorhabditis elegans. Ecotoxicol Environ Safe 111:66–71
Liang S, Yu ZY, Yin DQ (2015) Effects of sulfonamide mixtures at environmental concentrations on growth, feeding, catalase activity and the gene expression levels of Caenorhabditis elegans. Asian J Ecotoxicol 10:88–95 (In Chinese)
Yu ZY, Zhang J, Chen XX, Yin DQ, Deng HP (2013) Inhibitions on the behavior and growth of the nematode progeny after prenatal exposure to sulfonamides at environmental concentrations. J Hazard Mater 250–251:198–203
Brenner S (1974) The genetics of Caenorhabditis Elegans. Genetics 77:71–94
Li Z, Ai F, Zhang J, Yu Z, Yin D (2019) Using Caenorhabditis elegans for studying trans- and multi-generational effects of toxicants. J Vis Exp 149:e59367
Yu Z, Zhang J, Yin D (2016) Multigenerational effects of heavy metals on feeding, growth, initial reproduction and antioxidants in Caenorhabditis elegans. PLoS ONE 11:e0154529
Yu ZY, Sun GH, Liu YJ, Yin DQ, Zhang J (2017) Trans-generational influences of sulfamethoxazole on lifespan, reproduction and population growth of Caenorhabditis elegans. Ecotoxicol Environ Safe 135:312–318
Yu Z, Yin D, Zhang J (2019) Sex-dependent effects of sulfamethoxazole exposure on pro-/antioxidant status with stimulation on growth, behavior and reproduction in the amphipod Hyalella azteca. Environ Pollut 244:398–404
Zhang W, Chen L, Liu K, Chen L, Lin K, Chen Y, Yan Z (2014) Bioaccumulation of decabromodiphenyl ether (BDE209) in earthworms in the presence of lead (Pb). Chemosphere 106:57–64
Gluckman PD, Hanson MA, Beedle AS (2007) Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol 19:1–19
Liao VH-C, Li W-H, Shi Y-C, Tseng I-L (2013) Protective efficacy of selenite against lead-induced neurotoxicity in Caenorhabditis elegans. Toxicol Lett 221:S172
Wu Q, Liu P, Li Y, Du M, Xing X, Wang D (2012) Inhibition of ROS elevation and damage to mitochondrial function prevents lead-induced neurotoxic effects on structures and functions of AFD neurons in Caenorhabditis elegans. J Environ Sci 24:733–742
Zou R-X, Gu X, Ding J-J, Wang T, Bi N, Niu K, Ge M, Chen X-T, Wang H-L (2020) Pb exposure induces an imbalance of excitatory and inhibitory synaptic transmission in cultured rat hippocampal neurons. Toxicol In Vitro 63:104742
Zezza D, Tait S, Salda LD, Amorena M, Merola C, Perugini M (2019) Toxicological, gene expression and histopathological evaluations of environmentally realistic concentrations of polybrominated diphenyl ethers PBDE-47, PBDE-99 and PBDE-209 on zebrafish embryos. Ecotoxicol Environ Safe 183:109566
Soubry A (2018) Epigenetics as a driver of developmental origins of health and disease: did we forget the fathers? BioEssays 40:1700113
Araujo GS, Abess DMS, Soares AMVM, Loureiro S (2019) Multi-generational exposure to Pb in two monophyletic Daphnia species: individual, functional and population related endpoints. Ecotoxicol Environ Safe 173:77–85
Araujo GS, Abessa DMS, Soares AMVM, Loureiro S (2019) Multi-generational effects under single and pulse exposure scenarios in two monophyletic Daphnia species. Sci Total Environ 697:134031
Devóz PP, Gomes WR, De Araújo ML, Ribeiro DL, Pedron T, Antunes LMG, Batista BL, Barbosa F Jr, Barcelos GRM (2017) Lead (Pb) exposure induces disturbances in epigenetic status in workers exposed to this metal. J Toxicol Environ Health A 80:1098–1105
Montrose L, Faulk C, Francis J, Dolinoy DC (2017) Perinatal lead (Pb) exposure results in sex and tissue-dependent adult DNA methylation alterations in murine IAP transposons. Environ Mol Mutagen 58:540–550
Lai Y, Lu M, Gao X, Wu H, Cai Z (2011) New evidence for toxicity of polybrominated diphenyl ethers: DNA adducts formation from quinone metabolites. Environ Sci Technol 45:10720–10727
Zhao J, Xu T, Yin D, Zhang B, Bai J (2017) The regulatory roles of microRNA in effects of 2,2′4,4′-tetrabromodiphenyl Ether (BDE47) on the transcriptome of zebrafish larvae. PLoS ONE 12:e0169599
Fitsanakis VA, Aschner M (2005) The importance of glutamate, glycine, and γ-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharm 204:343–354
Wirbisky SE, Weber GJ, Lee JW, Cannon JR, Freeman JL (2014) Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol Lett 229:1–8
Brahma N, Gupta A (2020) Acute toxicity of lead in fresh water bivalves Lamellidens jenkinsianus obesa and Parreysia (Parreysia) corrugata with evaluation of sublethal effects on acetylcholinesterase and catalase activity, lipid peroxidation, and behavior. Ecotoxicol Environ Safe 189:109939
Acknowledgements
Not applicable.
Funding
This study was funded by National Science and Technology Project for Water Pollution Control and Treatment (2017ZX07201004) and International Science & Technology Cooperation Program of China (No. 2016YFE0123700).
Author information
Authors and Affiliations
Contributions
JZ performed the overall experiments and wrote the draft of the manuscript. ZY designed the experiment and modified the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
The effects of individual Pb or BDE-47 (A, C) and their sequential exposure (B, D) on the activities of AChE (A, B) and the contents of GABA (C, D) in Caenorhabditis elegans. The sequential exposure was a 12 h-exposure to BDE47 and another 12 h exposure to Pb (or vice versa). Blank column: exposure effects; Shadow column: recovery effects; *Significantly different from the control, p < 0.05. Data represent the means and standard error. Figure S2. The effects of individual Pb or BDE-47 (A, C) and their sequential exposure (B, D) on the activities of AChE (A, B) and the contents of GABA (C, D) in Caenorhabditis elegans offspring (F1). The sequential exposure was an early exposure to BDE47 in the parent and a later exposure to Pb in the offspring (or vice versa). Blank column: exposure effects; Shadow column: recovery effects; *Significantly different from the control, p < 0.05. Data represent the means and standard error.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Yu, Z. Transgenerational effects of different sequential exposure to 2,2′,4,4′-tetra-brominated diphenyl ether (BDE47) and lead (Pb) on Caenorhabditis elegans. Environ Sci Eur 32, 44 (2020). https://doi.org/10.1186/s12302-020-00318-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-00318-5