Abstract
Purpose
To quantify how often the LI-RADS v2018 category changed when utilizing major features only, when utilizing major and ancillary features, and when utilizing major and ancillary features excluding gadoxetate-specific ancillary features.
Methods
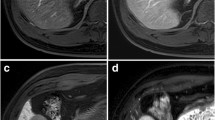
Retrospective analysis of 100 patients age 18 and older at high risk for hepatocellular carcinoma who had an MRI abdomen performed with intravenous contrast gadoxetate between 1/1/2017 and 3/23/2018. Each examination was reviewed by a body fellowship-trained radiologist. LI-RADS category was assigned to the liver observation after review of major features only. Ancillary features were then reviewed and LI-RADS category assigned both including and excluding ancillary features specific to gadoxetate.
Results
Utilizing all MRI ancillary features, including those specific to gadoxetate, changed the final LI-RADS category in 56.4% of liver observations, the majority an increase or decrease from LR-3. When not including the ancillary features specific to gadoxetate, the final LI-RADS category changed in 30.9% of observations, the majority increasing from LR-3 to LR-4.
Conclusion
Utilizing LI-RADS v2018 ancillary features can significantly alter the final LI-RADS category, especially when using gadoxetate-specific ancillary features. Understanding the correct application of ancillary features for the final LI-RADS category helps implement a more consistent category assessment amongst users.












Similar content being viewed by others
References
Bray, F, Ferlay J, Soerjomataram I (2018) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer Journal for Clinicians 68(6):394-424. https://doi.org/10.3322/caac.21492
Elsayes KM, Kielar AZ, Elmohr MM, et al. (2018) White Paper of the Society of Abdominal Radiology Hepatocellular Carcinoma Diagnosis Disease-Focused Panel on LI-RADS v2018 for CT and MRI. Abdominal Radiology 43(10):2625-2642. https://doi.org/10.1007/s00261-018-1744-4
Kielar AZ, Elsayes KM, Cherynak V, et al. (2018) LI-RADS Version 2018: What Is New and What Does This Mean to My Radiology Reports? Abdominal Radiology 44(1):41-42. https://doi.org/10.1007/s00261-018-1730-x
Chernyak V, Fowler K, Kamaya A, et al. (2018) Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 289(3):816-830. https://doi.org/10.1148/radiol.2018181494
American College of Radiology. Liver Imaging Reporting and Data System version 2018. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed July 2018.
Elsayes KM, Hooker JC, Agrons MM, et al. (2017) 2017 Version of LI-RADS for CT and MR Imaging: An Update. RadioGraphics 37(7):1994-2017. https://doi.org/10.1148/rg.2017170098
Cerny M, Cherynak V, Olivie D, et al. (2018) LI-RADS Version 2018 Ancillary Features at MRI. RadioGraphics 38(7):1973-2001. https://doi.org/10.1148/rg.2018180052
Cerny M, Bergeron C, Billiard J, et al. (2018) LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features. Radiology 288(1):118–128. https://doi.org/10.1148/radiol.2018171678
Joo I, Lee JM, Lee DH, et al. (2017) Liver Imaging Reporting and Data System v2014 Categorization of Hepatocellular Carcinoma on Gadoxetic Acid-Enhanced MRI: Comparison with Multiphasic Multidetector Computed Tomography. Journal of Magnetic Resonance Imaging 45(3):731-740. https://doi.org/10.1002/jmri.25406
Mitchell DG, Bashir MR, Sirlin CB (2018) Management implications and outcomes of LI-RADS-2, -3, -4, and -M category observations. Abdominal Radiology 43:143-148. https://doi.org/10.1007/s00261-017-1251-z
Acknowledgements
A special thanks is given to the Research Manager Carissa Walter within the Radiology Department as well as the Senior Research Analyst Lauren Clark with the Biostatistics and Data Science Department at the University of Kansas Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No disclosures for any author listed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boatright, C., Peterson, J., Williams, V.L. et al. LI-RADS v2018: utilizing ancillary features on gadoxetate-enhanced MRI to modify final LI-RADS category. Abdom Radiol 45, 3136–3143 (2020). https://doi.org/10.1007/s00261-020-02479-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02479-6