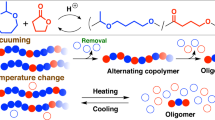
Abstract
We designed an N-phenylmaleimide derivative bearing an activated NHS ester (NHS-PhMI) for radical copolymerization with tert-butoxystyrene (tBOS) to achieve both precise alternating sequence control and functionalization. Essential to the monomer design is the electron-deficient carbon-carbon double bond for precise alternating propagation as well as the facile substitution reaction for the activated ester pendant. The precision of alternating sequences was evaluated by MALDI-TOF-MS analysis in comparison with a series of copolymers. Some amine compounds were quantitatively incorporated onto the maleimide side chain through the aminolysis reaction, whereas the tBOS units were transformed into vinylphenol counterparts via deprotection under acidic conditions. The resultant vinylphenol-based alternating copolymers showed unique solubilities and thermal properties depending on the substituent of the maleimide units.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lutz JF. Sequence-controlled polymerizations: the next Holy Grail in polymer science? Polym Chem. 2010;1:55–62.
Lutz JF, Ouchi M, Liu DR, Sawamoto M. Sequence-controlled polymers. Science. 2013;341:1238149.
Ouchi M, Sawamoto M. Sequence-controlled polymers via reversible-deactivation radical polymerization. Polym J. 2018;50:83–94.
Wagner-Jauregg T. The additional hetero polymerisation. Ber Dtsch Chem Ges B. 1930;63:3213–24.
Klumperman B. Mechanistic considerations on styrene-maleic anhydride copolymerization reactions. Polym Chem. 2010;1:558–62.
Hirooka M, Yabuuchi H, Iseki J, Nakai Y. Alternating copolymerization through the complexes of conjugated vinyl monomers-alkylaluminum halides. J Polym Sci Part A Polym Chem. 1996;34:2269–84.
Tesch M, Hepperle JAM, Klaasen H, Letzel M, Studer A. Alternating copolymerization by nitroxide-mediated polymerization and subsequent orthogonal functionalization. Angew Chem Int Ed. 2015;54:5054–9.
Terada S, Matsumoto A. Role of N-substituents of maleimides on penultimate unit effect for sequence control during radical copolymerization. Polym J. 2019;51:1137–46.
Oh D, Furuya Y, Ouchi M. Unusual radical copolymerization of suprabulky methacrylate with N-Hydroxysuccinmide acrylate: facile syntheses of alternating-rich copolymers of methacrylic acid and N-Alkyl acrylamide. Macromolecules. 2019;52:8577–86.
Zhao E, Lam JWY, Meng LM, Hong Y, Deng HQ, Bai GX, et al. Poly (maleic anhydride)-alt-(vinyl acetate): a pure oxygenic nonconjugated macromolecule with strong light emission and solvatochromic effect. Macromolecules. 2015;48:64–71.
Saha B, Bauri K, Bag A, Ghorai PK, De P. Conventional fluorophore-free dual pH- and thermo-responsive luminescent alternating copolymer. Polym Chem. 2016;7:6895–6900.
Huang J, Geng X, Peng C, Grove TZ, Turner SR. Enhanced fluorescence properties of stilbene-containing alternating copolymers. Macromol Rapid Commun. 2018;39:1700530.
O’Shea JP, Solovyeva V, Guo XR, Zhao JP, Hadjichristidis N, Rodionov VO. Sequence-controlled copolymers of 2,3,4,5-pentafluorostyrene: mechanistic insight and application to organocatalysis. Polym Chem. 2014;5:698–701.
Ishido Y, Aburaki R, Kanaoka S, Aoshima S. Well-defined alternating copolymers of benzaldehydes with vinyl ethers: precision synthesis by cationic copolymerization and quantitative degradation to cinnamaldehydes. Macromolecules. 2010;43:3141–4.
Hill MR, Guegain E, Tran J, Figg CA, Turner AC, Nicolas J, et al. Radical ring-opening copolymerization of cyclic ketene acetals and maleimides affords homogeneous incorporation of degradable units. ACS Macro Lett. 2017;6:1071–7.
Ouchi M, Nakano M, Nakanishi T, Sawamoto M. Alternating sequence control for carboxylic acid and hydroxy pendant groups by controlled radical cyclopolymerization of a divinyl monomer carrying a cleavable spacer. Angew Chem Int Ed. 2016;55:14584–9.
Cazares-Cortes E, Baker BC, Nishimori K, Ouchi M, Tournilhac F. Polymethacrylic acid shows thermoresponsivity in an organic solvent. Macromolecules. 2019;52:5995–6004.
Kametani Y, Sawamoto M, Ouchi M. Control of the alternating sequence for n-isopropylacrylamide (NIPAM) and methacrylic acid units in a copolymer by cyclopolymerization and transformation of the cyclopendant group. Angew Chem Int Ed. 2018;57:10905–9.
Nishimori K, Cazares-Cortes E, Guigner JM, Tournilhac F, Ouchi M. Physical gelation of AB-alternating copolymers made of vinyl phenol and maleimide units: cooperation between precisely incorporated phenol and long alkyl pendant groups. Polym Chem. 2019;10:2327–36.
Tanaka K, Waki H, Ido Y, Akita S, Yoshida Y, Yoshida T, et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time‐of‐flight mass spectrometry. Rapid Commun Mass Spectrom. 1988;2:151–3.
Nishimori K, Sawamoto M, Ouchi M. Design of maleimide monomer for higher level of alternating sequence in radical copolymerization with styrene. J Polym Sci Part A Polym Chem. 2019;57:367–75.
Leonard NM, Brunckova J. In situ formation of N-trifluoroacetoxy succinimide (TFA-NHS): one-pot formation of succinimidyl esters, N-trifluoroacetyl amino acid succinimidyl esters, and N-maleoyl amino acid succinimidyl esters. J Org Chem. 2011;76:9169–74.
Li BN, Liang JF. A linker approach to phospholipopeptides. Eur J Lipid Sci Tech. 2016;118:273–8.
Das A, Theato P. Activated ester containing polymers: opportunities and challenges for the design of functional macromolecules. Chem Rev. 2016;116:1434–95.
Nishimori K, Ouchi M, Sawamoto M. Sequence analysis for alternating copolymers by MALDI-TOF-MS: importance of initiator selectivity for comonomer pair. Macromol Rapid Commun. 2016;37:1414–20.
Mishima E, Yamago S. Controlled alternating copolymerization of (Meth)acrylates and Vinyl ethers by using organoheteroatom-mediated living radical polymerization. Macromol Rapid Commun. 2011;32:893–8.
Mishima E, Tamura T, Yamago S. Controlled copolymerization of acrylate and 6-methyleneundecane by organotellurium-mediated living radical polymerization (TERP). Macromolecules. 2012;45:2989–94.
Srichan S, Chan-Seng D, Lutz JF. Influence of strong electron-donor monomers in sequence-controlled polymerizations. ACS Macro Lett. 2012;1:589–92.
ten Brummelhuis N, Weck M. Orthogonal multifunctionalization of random and alternating copolymers. ACS Macro Lett. 2012;1:1216–8.
Nakamura K, Hatakeyama T, Hatakeyama H. Effect of substituent groups on hydrogen-bonding of polyhydroxystyrene derivatives. Polym J. 1983;15:361–6.
Kuo SW, Tung PH, Chang FC. Syntheses and the study of strongly hydrogen- bonded poly(vinylphenol-b-vinylpyridine) diblock copolymer through anionic polymerization. Macromolecules. 2006;39:9388–95.
Degawa K, Matsumoto A. Retardation effect of catechol moiety during radical copolymerization of 3,4-dihydroxystyrene with various monomers. Chem Lett. 2019;48:928–31.
Acknowledgements
This work was supported by JSPS KAKENHI grants 17J06075 (KN), 17H06453 (MO) and 19H00911 (MO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nishimori, K., Ouchi, M. Design of a maleimide monomer to achieve precise sequence control and functionalization for an alternating copolymer with vinylphenol. Polym J 52, 717–729 (2020). https://doi.org/10.1038/s41428-020-0326-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0326-9