Abstract
Background and aims
While large differences in microbial biomass and extracellular enzyme activities (EEAs) between rhizosphere and bulk soils have been demonstrated, the potentially different response of microbes and their EEAs in rhizosphere and bulk soils to nitrogen (N) deposition is still not elucidated.
Methods
We analyzed the microbial biomass and EEAs in the rhizosphere and bulk soils of Sibiraea angustata in an alpine shrubland on the eastern Qinghai-Tibet Plateau after chronic N application. We also analyzed the stoichiometric linkages between plants, microbes, enzymes and soils to clarify the coupled responses of aboveground plants and belowground ecological processes.
Results
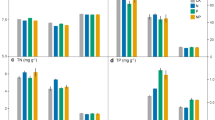
Microbial nutrient concentrations and activities of EAAs responded differently to N addition in the rhizosphere and bulk soils. In the rhizosphere, N addition caused a significant increase in microbial biomass carbon (C), N and phosphorus (P) concentrations and greater P-degrading enzyme activity (relative to the activities of C- and N-degrading enzymes), which induced a significant reduction in enzyme C:P and N:P ratios. The rhizosphere enzyme N:P ratio was negatively correlated with the N:P ratios of plant, soil and microbe, implying that increased plant and microbial P uptake under N addition may gradually aggravate rhizosphere P limitation. However, for the bulk soil, N addition did not affect microbial biomass but significantly enhanced C-degrading enzyme activity and decreased the enzyme C:N ratio. Meanwhile, the bulk-soil enzyme C:N ratio was negatively correlated with the soil C:N ratio but independent of the plant C:N ratio, implying that N addition may enhance bulk-soil microbial C limitation.
Conclusions
Our study suggests that elevated N deposition may induce differential microbial nutrient limitation between the rhizosphere and bulk soils due to the plant-microbe-soil interactions in the rhizosphere. This study highlights the importance of incorporating rhizosphere microbial processes into biogeochemical models describing environmental changes.




Similar content being viewed by others
References
Ågren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128(3):94–08
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37(5):937–944
Allison SD, Weintraub MN, Gartner TB, Waldrop MP (2011) Evolutionary ecosystem principles as regulators of soil enzyme production and ecosystem function. In: Shukla G, Varma A (Eds.), Soil Enzymology. Springer-Verlag, Berlin, Germany, pp. 229–243
Aneja MK, Sharma S, Fleischmann F, Stich S, Heller W, Bahnweg G et al (2006) Microbial colonization of beech and spruce litter – influence of decomposition site and plant litter species on the diversity of microbial community. Microb Ecol 52:127–135
Boot CM, Hall EK, Denef K, Baron JS (2016) Long-term reactive nitrogen loading alters soil carbon and microbial community properties in a subalpine forest ecosystem. Soil Biol Biochem 92:211–220
Bell C, Carrillo Y, Boot CM, Rocca JD, Pendall E, Wallenstein M (2014) Rhizosphere stoichiometry: are C : N : P ratios of plants, soils, and enzymes conserved at the plant species–level? New Phytol 201(2):505–517
Bennett JA, Klironmos J (2018) Mechanisms of plant–soil feedback: interaction among biotic and abiotic drivers. New Phytol 222(1):91–96
Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–45
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115(1):65–76
Carrara JE, Walter CA, Hawkins JS, Peterjohn WT, Averill C, Brzostek ER (2018) Interactions among plants, bacteria, and fungi reduce extracellular enzyme activities under long–term N fertilization. Glob Chang Biol 24(6):2721–2734
Chen H, Li D, Zhao J, Zhang W, Xiao K, Wang K (2018) Nitrogen addition aggravates microbial carbon limitation: evidence from ecoenzymatic stoichiometry. Geoderma 329:61–64
Cleveland CC, Liptzin D (2007). C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85(3):235–252
Corre MD, Veldkamp E, Arnold J, Wright SJ (2010) Impact of elevated N input on soil N cycling and losses in old–growth lowland and montane forests in Panama. Ecology 91:1715–1729
Corrales A, Turner BL, Tedersoo L, Anslan S, Dalling JW (2017) Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol 27:14–23
Cui Y, Bing H, Fang L, Jiang M, Shen G, Yu J, Wang X, Zhu H, Wu Y, Zhang X (2019) Extracellular enzyme stoichiometry reveals the carbon phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil:1–14. https://doi.org/10.1007/s11104-019-04159-x
Cusack D, Silver W, Torn M, Burton S, Firestone M (2011) Changes in microbial community characteristics with nitrogen addition and effects on soil organic matter in two tropical forests. Ecology 92:1–33
Dalmonech D, Lagomarsino A, Moscatelli MC, Chiti T, Valentini R (2010) Microbial performance under increasing nitrogen availability in a Mediterranean forest soil. Soil Biol Biochem 42:1596–1606
Deng Q, Hui D, Dennis S, Reddy KC (2017) Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition : a meta–analysis. Global Ecology and Biogerography 26:713–728
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC (2013) Stoichiometry constrains microbial response to root exudation—insights from a model and a field experiment in a temperate forest. Biogeosciences 10(2):821–838
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fanin N, Moorhead D, Bertrand I (2016) Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 129:21–36
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21(5):2082–2094
Freedman ZB, Romanowicz KJ, Upchurch RA, Zak D (2015) Differential responses of total and active soil microbial communities to long–term experimental N deposition. Soil Biol Biochem 90:275–282
Fu G, Shen Z-X (2017) Response of alpine soils to nitrogen addition on the Tibetan plateau: a meta-analysis. Appl Soil Ecol 114:99–104
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science (New York, N.Y.) 320(5878):889–892
Goll DS, Brovkin V, Parida BR et al (2012) Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 9:3547–3569
He W, Yang X, Xiao J, Zhang Z, Jiang Z, Yuan Y et al (2017) Effects of nitrogen enrichment on root exudative carbon inputs in Sibiraea angustata shrubbery at the eastern fringe of the Qinghai–Xizang plateau. Chin J Plant Ecol 41(6):610–621 (in Chinese with English abstract)
Hiltner L (1904) Über neuere Erfahrungen und Probleme auf dem Gebiete der Bodenbakteriologie unter besonderer Berücksichtigung der Gründüngung und Brache. Arbeiten der Deutschen Landwirtschaftlichen Gesellschaft 98:59–78
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Jian S, Li J, Chen J et al (2016) Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization : a meta–analysis. Soil Biol Biochem 101:32–43
Jiang J, Zong N, Song M, Shi P, Ma WL, Fu G, Shen Z, Zhang X, Ouyang H (2013) Responses of ecosystem respiration and its components to fertilization in an alpine meadow on the Tibetan plateau. Eur J Soil Biol 56:101–106
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Keeler BL, Hobbie SE, Kellogg LE (2008) Effects of long–term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165(4):382–396
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198(3):656–669
Li Y, Niu S, Yu G (2016) Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta–analysis. Glob Chang Biol 22(2):934–943
Liu G (1996) Standard methods for the observation and analysis of Chinese ecosystem research network: soil analysis and profile description. Standards Press of China, Beijing (in Chinese)
Liu LL, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828
Yu G, Jia Y, He N, Zhu J, Chen Z, Wang Q et al (2019) Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat Geosci 12(6):424–429
Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta–analysis. Agric Ecosyst Environ 140(1–2):234–244
Mack MC, Schuur EAG, Bret–Harte MS, Shaver GR, Chapin FS (2004) Ecosystem carbon storage in arctic tundra reduced by long–term nutrient fertilization. Nature 431:440–443
Marklein A, Houlton B (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193(3):696–704
Moreau D, Barggett R, Finlay RD, Jones DL, Phillippot L (2019) A plant perspective on nitrogen cycling in the rhizosphere. Funct Ecol 33:540–552
Midgley MG, Phillips RP (2016) Resource stoichiometry and the biogeochemical consequences of nitrogen deposition in a mixed deciduous forest. Ecology 97(12):3369–3378
Mooshammer M, Wanek W, Hämmerle L et al (2014a) Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Mooshammer M, Wanek W, Zechmeister–Boltenstern S, Richter A (2014b) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol Biochem 53:133–141
Ostonen I, Truu M, Helmisaari H-S, Lukac M, Borken W, Vanguelova E, Godbold DL, Lõhmus K, Zang U, Tedersoo L, Preem JK, Rosenvald K, Aosaar J, Armolaitis K, Frey J, Kabral N, Kukumägi M, Leppälammi-Kujansuu J, Lindroos AJ, Merilä P, Napa Ü, Nöjd P, Parts K, Uri V, Varik M, Truu J (2017) Adaptive root foraging strategies along a boreal-temperate forest gradient. New Phytol 215:977–991
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long–term CO2 fumigation. Ecol Lett 14:187–194
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18(6):1918–1927
Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer N (2010) Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 91:3463–3470
Rappe–George MO, Choma M, Čapek P, Börjesson G, Kaštovská E, Šantrůčková H, Gärdenäs AI (2017) Indications that long–term nitrogen loading limits carbon resources for soil microbes. Soil Biol Biochem 115:310–321
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1(7):430–437
Saiya–Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sterner RW, Elser JJ (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton, NJ.
Thomas RQ, Canham CD, Weathers KC, Goodale CL (2010) Increased tree carbon storage in response to nitrogen deposition in the US. Nat Geosci 3:13–17
Thomas RQ, Bonan GB, Goodale CL (2013) Insights into mechanisms governing forest carbon response to nitro– gen deposition: a model–data comparison using observed responses to nitrogen addition. Biogeosciences 10:3869–3887
Treseder KK (2008) Nitrogen addition and microbial biomass: a meta–analysis of ecosystem studies. Ecol Lett 1:1111–1120
Turner BL, Wright JS (2013) The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 117(1):115–130
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14(4):1172–1177
Wang D, He H, Gao Q, He W, Zhao C, Yin H, Liu Q (2017) Effects of short–term N addition on plant biomass allocation and C and N pools of the Sibiraea angustata scrub ecosystem. Eur J Soil Sci 68(2):212–220
Wang C, Lu X, Mori T, Mao Q, Zhou K, Zhou G et al (2018) Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol Biochem 121:103–112
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemsitry 117:101–113
Wu J, He Z, Wei W, O’Donnell AG, Syers JK (2000) Quantifying microbial biomass phosphorus in acid soils. Biol Fertil Soils 32(6):500–507
Wu N (1998) The community types and biomass of Sibiraea angustata scrub and their relationship with environment factors in northwestern Sichuan. Acta Bot Sin 40:860–870
Yang Y, Fang J, Smith P, Tang Y, Chen A, Ji C et al (2009) Changes in topsoil carbon stock in the Tibetan grasslands between the 1980s and 2004. Glob Chang Biol 15(11):2723–2729
Yin H, Li Y, Xiao J, Xu Z, Cheng X, Liu Q (2013) Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Glob Chang Biol 19(7):2158–2167
Yue K, Fornara DA, Yang W, Peng Y, Li Z, Wu F, Peng C (2017) Effects of three global change drivers on terrestrial C:N:P stoichiometry: a global synthesis. Glob Chang Biol 23(6):2450–2463
Zadworny M, McCormack ML, Z ˇytkowiak R, Karolewski P, Mucha J, Oleksyn J (2017) Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob Change Biol 23:1218–1231
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol Monogr 85:133–155
Zhao W, Reich PB, Yu Q, Zhao N, Yin C, Zhao C et al (2018) Shrub type dominates the vertical distribution of leaf C : N : P stoichiometry across an extensive altitudinal gradient. Biogeosciences 15(7):2033–2053
Zhou Z, Wang C, Zheng M, Jiang L, Luo Y (2017) Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol Biochem 115:433–441
Acknowledgments
This study was supported jointly by the Priority Research Program of Frontier Science, Chinese Academy of Science (QYZDB-SSW-SMC023), the National Natural Science Foundation of China (No. 31670449, 31872700), and the Sichuan Key R & D Program (21018SZ0336). We are also grateful for the funding provided by the Ecological Security and Protection Key Laboratory of Sichuan Province, Mianyang Normal University (ESP1702).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth M Baggs.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Zhu, X., Liu, M., Kou, Y. et al. Differential effects of N addition on the stoichiometry of microbes and extracellular enzymes in the rhizosphere and bulk soils of an alpine shrubland. Plant Soil 449, 285–301 (2020). https://doi.org/10.1007/s11104-020-04468-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04468-6