Abstract
Background
Several factors contribute to neurodevelopmental outcomes in preterm infants. The aim of this study was to examine the genetic and environmental influences on long-term outcomes in preterm twins.
Methods
From a prospective cohort of 225 preterm neonates studied with MRI, 24 monozygotic and 52 dizygotic twins were included. Neurodevelopmental outcomes at 1.5 and 3 years were assessed with the Bayley-III and at 4.5 years with The Movement Assessment Battery for Children and The Wechsler Preschool and Primary Scale of Intelligence-III.
Results
Preterm monozygotic and dizygotic twin pairs (N = 76 neonates) had similar neurodevelopmental outcomes at all time points. Monozygotic twins (N = 24) did not show greater agreement for outcomes relative to dizygotic twins (N = 52). Twin pairs who were discordant in development (N = 12) were born at a lower gestational age and had a higher incidence of bronchopulmonary dysplasia and retinopathy of prematurity. Discordant twins become more similar in cognitive and language outcomes over time.
Conclusions
Neurodevelopmental outcomes in preterm twins may relate more strongly to environmental factors than genetics. Discordant twins were born earlier and had more perinatal morbidities. Despite the initial discordance, these twin pairs become similar in outcomes over time, which may reflect the positive impact of home environment or early intervention programs.
Impact
-
Neurodevelopmental outcomes in preterm twins relate more strongly to environmental factors than genetics.
-
Monozygotic twins did not show greater agreement in outcomes relative to dizygotic twins suggesting a stronger environmental, rather than genetic, influence on development.
-
Twin pairs who were discordant in development were born at a lower gestational age and had a higher incidence of perinatal morbidities.
-
Despite the initial discordance, these twin pairs become more similar in cognitive and language outcomes over time, which may reflect the positive impact of early intervention programs or home environment.
-
Neurodevelopmental outcomes in preterm twins are influenced by exposure to early-life insults or environmental stressors.
-
The initial variability in outcomes among preterm infants is not fixed, and efforts made post-discharge from the neonatal intensive care unit can have a substantial impact on long-term outcomes.
Similar content being viewed by others
Introduction
Preterm birth is defined as birth before 37 completed weeks of gestation and is accompanied by a spectrum of morbidities and brain injuries. The risk of preterm birth is increased with multiple gestations, young or advanced maternal age, previous preterm delivery, and maternal and fetal complications.1 Genetic factors may also contribute to preterm birth. A recent genome-wide association study identified variants at six gene loci that were associated with gestational duration and preterm labor.2 Previous studies have shown that preterm infants are at an increased risk of adverse neurodevelopmental outcomes, including poor cognitive, language and motor development, hearing and vision impairments, and brain injury.3,4,5,6,7 Risk factors associated with poor neurodevelopmental outcomes in preterm neonates include low gestational age, low birth weight, small head circumference, bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), infection, frequent invasive procedures, and brain injury.5,7,8,9,10,11,12,13 Brain injury that can occur in the neonatal period includes white matter injury, intraventricular hemorrhage, and cerebellar hemorrhage. These preterm-birth related morbidities may be present at birth or acquired during the neonatal period. While some of these risk factors may have a genetic contribution (e.g., BPD, ROP),14,15 many of these risk factors are acquired insults.
In recent years, there has been an increase in multiple births in Canada, with twin and triplet births accounting for 2.2% of all live births in 1995 and 3.2% in 2012.16,17 This recent increase in multiple births has been attributed to older maternal age at conception, use of ovulation induction agents, and the use of assisted reproductive technology.18,19,20 Multiple gestation is a risk factor for preterm birth resulting in either spontaneous preterm labor or indicated preterm delivery for a maternal or fetal condition.1,21,22 In Canada, 57% of twins and 96% of higher-order multiple births were born preterm.22 Similarly, a large multicentre study in the United States by Gardner and colleagues found that 54% of twins were born preterm compared with 9.6% among singletons. While studies have examined neurodevelopmental outcomes among preterm singletons, longitudinal studies of preterm twins and higher order multiples are limited.23,24,25,26,27 Twin studies offer the advantage of studying the genetic and environmental contribution to a given trait. If monozygotic (MZ) twin pairs have greater agreement for the trait relative to dizygotic (DZ) pairs, this suggests a strong genetic contribution. However, if MZ and DZ twin pairs have similar levels of agreement, then environmental factors may have a stronger influence.
The objective of the present study was to examine the genetic and environmental influences on long-term neurodevelopmental outcomes in preterm MZ and DZ twins. Our specific aims were (1) to assess agreement among preterm twin pairs for perinatal morbidities that are recognized to have a negative impact on neurodevelopment (BPD, ROP, infection, and brain injury) and (2) to examine agreement in motor, cognitive, and language outcomes among MZ and DZ twin pairs and twin pairs concordant or discordant for neurodevelopment at 1.5, 3, and 4.5 years. We hypothesized that preterm MZ twin pairs will have greater agreement for perinatal morbidities and outcomes relative to DZ twin pairs due to their identical genetic makeup.
Methods
Participants
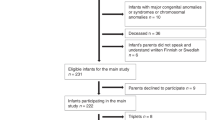
Study participants were selected from a prospective cohort of preterm infants born between 24 and 32 weeks’ gestational age from the tertiary-level neonatal intensive care unit (NICU) at the British Columbia’s Women’s Hospital as described previously.9 Neonates were excluded if they had congenital malformations, congenital infection, or ultrasound evidence of a large parenchymal hemorrhage (>2 cm). This preterm cohort included a total of 234 neonates born between April 2006 and August 2010. The final cohort for the present study comprised 24 MZ twins and 52 DZ twins. Inclusion as a twin in this study was based on placental data. All twins included in the study had placental data available to determine zygosity. There were two neonates in the overall cohort, who were single survivors of a twin gestation, and these two twin pregnancies were not included in our sample as there was only one surviving twin. This study was approved by the University of British Columbia Research Ethics Board. Informed consent was obtained from the parent or legal guardian.
Neonatal clinical data
Prospective medical chart review was conducted by an experienced neonatal research nurse. Clinical risk factors for adverse neurodevelopmental outcome were selected based on the strength of prior associations in this and other cohorts5,7,8,9,10,11,12 and included BPD (defined as requiring oxygen at 36 weeks), ROP, infection, and brain injury (white matter injury, intraventricular hemorrhage, and cerebellar hemorrhage).
Magnetic resonance imaging (MRI) scans
Neonates were scanned with MRI as soon as clinically stable after birth using an MRI-compatible incubator and a specialized neonatal head coil (Advanced Imaging Research, Cleveland, OH) on a Siemens 1.5 T Avanto scanner (Erlangen, Germany). A second MRI scan was acquired at term equivalent age. MRI scans were performed without pharmacological sedation. Of the 225 neonates in this cohort, 186 had two MRI scans, and 39 had only the first MRI scan (there were no neonates with only the second scan). Detailed imaging methods for this study cohort have been previously described.28 The MRI images were reviewed by an experienced neuroradiologist who was blind to the neonate’s medical history. Both the initial and the term-equivalent MRI scans were assessed for the presence of white matter injury, intraventricular hemorrhage, and cerebellar hemorrhage as reported previously; the maximum severity of injury was evident on the early scan and used in the analyses.29,30
Neurodevelopmental assessment
Children were assessed at 1.5 and 3 years with Bayley Scales of Infant and Toddler Development, Third edition (Motor, Cognitive, and Language Composite scores). Outcomes at 4.5 years was assessed with The Movement Assessment Battery for Children and The Wechsler Preschool and Primary Scale of Intelligence-III using Full Scale IQ [FSIQ] as the Cognitive outcome and Verbal IQ as the Language outcome score. Assessments were completed by experienced physiotherapy, occupational therapy, and psychology staff in the Neonatal Follow-up Program at BC Children’s & Women’s Health Centre, who had access to the participants’ clinical data but were blind to the MRI data. Inter-rater reliability measurements for neurodevelopmental outcomes were not obtained.
Data analysis
All statistical analyses were conducted using SPSS Statistics version 25. Clinical data for MZ and DZ twins were compared using unpaired t tests (gestational age, birth weight, head circumference) and chi-squared tests (BPD, ROP, infection, white matter injury, intraventricular hemorrhage, and cerebellar hemorrhage). Shared demographic factors (e.g., gestational age, maternal and paternal education) were compared for twin pairs. Unshared demographic factors (e.g., birth weight, head circumference, BPD, ROP, infection, and brain injury) were compared for all twins, rather than pairs. To estimate the degree of agreement for categorical clinical variables within twin pairs beyond that expected by chance, we calculated Cohen’s Kappa with values of 0 indicating no agreement, 0–0.20 as slight agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement, and 0.81–1 as high agreement.31
Percent score difference was calculated for MZ and DZ twin motor, cognitive, and language scores at 1.5, 3, and 4.5 years. For this analysis, twins were assessed as part of a pair and not individually. Twin pairs were classified as concordant or discordant based on their initial motor, cognitive, and language scores at 1.5 years. Discordance was defined as a score difference >2 standard deviation (SD) from the mean percent score difference for the motor, cognitive, or language scores. This cut-off was selected based on our previous data using Bland Altman plots, which identified outliers that were outside the 95% limits of agreement (>2 SD of the mean percent score difference for the group). For subsequent analyses, we defined discordance as a percent score difference >2 SD of the mean percent score difference of the group. Clinical characteristics (gestational age, birth weight, head circumference) and the presence of perinatal morbidities (BPD, ROP, infection, white matter injury, intraventricular hemorrhage, and cerebellar hemorrhage) were compared between concordant and discordant twins using unpaired t tests and chi-squared tests. Mean percent score differences for motor, cognitive, and language scores were generated for discordant and concordant twin groups at 1.5, 3, and 4.5 years.
Results
Clinical characteristics
Clinical characteristics for the study cohort are presented in Table 1 for 24 MZ twins and 52 DZ twins. MZ and DZ twins did not differ in sex, gestational age, birth weight, or head circumference. The presence of perinatal morbidities (BPD, ROP, infection, white matter injury, intraventricular hemorrhage, and cerebellar hemorrhage) was not significantly different between MZ and DZ twins. Assessments at 1.5 years were available for 20 MZ twins and 44 DZ twins (84% of initial sample). Assessments at 3 years were available for 24 MZ twins and 42 DZ twins (87% of initial sample). Assessments at 4.5 years were available for 18 MZ twins and 42 DZ twins (79% of initial sample).
Agreement for risk factors among MZ and DZ twin pairs
MZ twin pairs had substantial agreement for the presence or absence of ROP (κ = 0.63) and moderate agreement for BPD (κ = 0.46) and infection (κ = 0.33). MZ twins had moderate agreement for cerebellar hemorrhage (κ = 0.44) and slight agreement for white matter injury (κ = −0.18) and intraventricular hemorrhage (κ = −0.11).
DZ twin pairs had substantial agreement for the presence of ROP (κ = 0.94), moderate agreement for BPD (κ = 0.43), and fair agreement for infection (κ = 0.40). DZ twins had moderate agreement for intraventricular hemorrhage (κ = 0.42), fair agreement for cerebellar hemorrhage (κ = 0.26), and slight agreement for white matter injury (κ = 0.02).
Agreement in neurodevelopmental outcomes over time
Motor, language, and cognitive scores for MZ and DZ twin pairs are listed in Table 2. Both MZ and DZ twin pairs were similar in cognitive and language outcomes. Bayley-III cognitive scores were lower in MZ twins at the 3-year follow-up. For all other outcomes, there were no significant differences in scores between MZ and DZ twins (Table 2). When plotted, agreement in motor outcomes between twin pairs decreased over time as indicated by increasing motor score differences for both MZ and DZ twins (Fig. 1). Agreement in cognitive and language scores remained stable over time for both MZ and DZ twin pairs (Figs. 2 and 3).
Twin pairs discordant in neurodevelopmental outcomes
Twin pairs with percent score differences >2 SD of the mean score difference were classified as discordant (N = 12, 6 pairs). Discordant twins had a significantly lower gestational age, birth weight, and head circumference relative to concordant twins (Table 3). A greater proportion of discordant twins had BPD (χ2 = 8.0, P = 0.01) and ROP (χ2 = 5.9, P = 0.02). Within discordant twin pairs, there were two pairs with a birth weight difference >20%. In terms of brain injury, 50% (N = 6) of all discordant twins had brain injury. Brain injury involved minimal white matter injury (N = 1), severe white matter injury (N = 1), and intraventricular hemorrhage (N = 5). Within discordant twin pairs, there was 1 pair in which only the lower scoring twin had brain injury (severe white matter injury and intraventricular hemorrhage), 2 pairs in which both twins had brain injury (intraventricular hemorrhage), 1 pair in which the higher scoring twin had brain injury (minimal white matter injury and intraventricular hemorrhage), and 2 pairs with no brain injury in either twin.
Motor, language, and cognitive scores between discordant and concordant twin pairs are listed in Table 4. Discordant twins had lower cognitive scores at 1.5-year follow-up and lower motor scores at 3-year follow-up relative to concordant twins. There were no significant differences in the other assessments between discordant and concordant twin pairs (Table 4). Agreement in motor outcomes between twin pairs decreased over time for both discordant and concordant twin pairs, as indicated by increasing motor score differences (Fig. 4). Discordant twin pairs had increasing agreement for cognitive and language outcomes as indicated by decreasing score differences over time (Figs. 5 and 6).
Discussion
In our cohort of preterm twins, MZ and DZ twin pairs had similar long-term neurodevelopmental outcomes through early childhood. MZ twins did not show greater agreement in outcomes relative to DZ twins suggesting a stronger environmental, rather than genetic, influence on development. Preterm infants may have neurodevelopmental outcomes based on their exposure to early-life insults or environmental stressors rather than their genetic makeup. Differences in the presence of perinatal morbidities such as BPD, ROP, infection, and brain injury may lead to different outcomes in two genetically similar twins. In our study, twin pairs who were discordant in neurodevelopmental outcomes were born earlier with a lower birth weight and higher incidence of BPD and ROP relative to concordant twins. The mechanism underlying differential susceptibility to perinatal morbidities within twin pairs remains unclear. One twin may have had a compromised intrauterine development, difficult extraction, or lower birth weight relative to the other twin. These differences may cause one twin to be more vulnerable to perinatal morbidities such as BPD, ROP, infection, and brain injury, which in turn will lead to differences in neurodevelopmental outcome.
One third of preterm twins in our sample had brain injury detected on their neonatal MRI. It is well established that these perinatal morbidities can have a negative impact on neurodevelopment with worse outcomes being associated with an increasing number of these insults.3 The presence of neonatal brain injury has been shown to be a significant predictor of adverse neurodevelopmental outcome, including poor cognitive, language and motor development and an increased risk of cerebral palsy.6,9,30 When we examined motor outcomes over time, differences in motor scores increased over time for all groups (MZ, DZ, discordant, and concordant). This may be due to motor impairments in children who had sustained a brain injury becoming more apparent with increasing age and abilities.
When discordant twin pairs were followed over time, initial differences were minimized, and pairs become more similar in cognitive and language abilities. The lower scoring twin in the discordant pair appears to catch up, which may reflect the positive impact of early intervention programs or home environment. Developmental surveillance in preterm infants allows for early detection of delays and implementation of allied health services, such as physical therapy, occupational therapy, and speech language pathology. A recent systematic review found that early intervention programs for preterm infants have a positive impact on neurodevelopmental outcomes during infancy with cognitive benefits persisting into preschool age.32 Other environmental factors such as parental education, socioeconomic status, and parenting style have also been shown to be associated with neurodevelopmental outcomes in preterm populations.33,34,35,36 For instance, higher parental education is associated with increased cognitive and language scores at 18 months among preterm children.33 Similarly, a recent study found that cognitive outcomes at preschool age was associated with maternal education, with greater cognitive scores in children whose mothers had a post-secondary degree relative to children whose mothers had lower levels of education.36 Interestingly, this study also reported that the adverse cognitive outcomes associated with perinatal brain injury were mitigated in preterm children born to mothers with higher education.36 Other parenting behaviors such as positive affect, sensitivity, and parent–child synchrony have also been shown to be associated with better cognitive and social development in children born preterm.35 Our findings along with previous research indicates that the initial variability in outcomes among preterm infants is not fixed, and efforts made post-discharge from the NICU can have a substantial impact on long-term outcomes.
The strengths of this study include the high subject retention at follow-up assessments. We also had an adequate sample size of 76 twins for an MRI study, which is comparable to previous MRI studies of this age group.37,38 There are limitations of this study that should be acknowledged. Zygosity was determined based on placental pathology and was not confirmed with genetic testing. We had a small number of discordant twins relative to concordant twins. Neurodevelopment outcomes at all three time points cannot be assessed with the same scales. The Bayley-III subscales may not be directly comparable to the Movement Assessment Battery for Children and The Wechsler Preschool and Primary Scale of Intelligence-III. We recognize that increasing motor score differences may reflect the use of different measures. Our preterm twins were followed only until 4.5 years of age, and any developmental problems that become evident after this age would not have been captured. Although preterm twin pairs usually share similar postnatal home and family environments (e.g., family socioeconomic status, parental education), differences in care within pairs by family members or by teachers at daycare could not be controlled and may have contributed to differences in outcomes. We do not have data on the number of illnesses that twins acquired and differences in illness severity, and number of illnesses may have impacted neurodevelopmental outcomes between twin pairs. Interventions after discharge from the NICU were not directly examined; however, infant development programs and physiotherapy are available province-wide through government services to all developmentally high-risk infants and toddlers.
In our cohort of preterm twins, MZ and DZ twin pairs had similar outcomes through early childhood suggesting that neurodevelopmental outcomes may relate more strongly to environmental rather than genetic factors. Lower gestational age and a higher incidence of perinatal morbidities were associated with discordant development among twin pairs. Over time, discordant pairs become more similar in cognitive and language outcomes, which may reflect the positive impact of early intervention programs or home environment. These findings are encouraging; however further studies are needed to better understand the susceptibility for perinatal illness and factors contributing to discordant development in preterm twins.
References
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Zhang, G. et al. Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med. 377, 1156–1167 (2017).
Manuck, T. A., Sheng, X., Yoder, B. A. & Varner, M. W. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am. J. Obstet. Gynecol. 210, 426.e1–426.e9 (2014).
Mwaniki, M. K., Atieno, M., Lawn, J. E. & Newton, C. R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379, 445–452 (2012).
Teune, M. J., van Wassenaer, A. G., van Dommelen, P., Mol, B. W. J. & Opmeer, B. C. Perinatal risk indicators for long-term neurological morbidity among preterm neonates. Am. J. Obstet. Gynecol. 204, 396.e1–396.e14 (2011).
Wood, N. S., Marlow, N., Costeloe, K., Gibson, A. T. & Wilkinson, A. R. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N. Engl. J. Med. 343, 378–384 (2000).
Schlapbach, L. J. et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 12, 198 (2012).
Chau, V. et al. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr. Res. 71, 274–279 (2012).
Chau, V. et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81, 2082–2089 (2013).
Schmidt, B. et al. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J. Pediatr. 167, 982.e2–986.e2 (2015).
Cheong, J. L. Y. et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 121, e1534–e1540 (2008).
Kersbergen, K.J. et al. Relation between clinical risk factors, early cortical changes, and neurodevelopmental outcome in preterm infants. Neuroimage 142, 301–310 (2016).
Vinall, J. & Grunau, R. E. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr. Res. 75, 584–587 (2014).
Bhandari, V. & Gruen, J. R. The genetics of bronchopulmonary dysplasia. Semin. Perinatol. 30, 185–191 (2006).
Bizzarro, M. J. et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics 118, 1858–1863 (2006).
Statistics Canada. Trends in Canadian births, 1992 to 2012. http://www.statcan.gc.ca/pub/82-625-x/2016001/article/14314-eng.htm (2016).
Statistics Canada HSD. Births 2008. http://www.statcan.gc.ca/access_acces/archive.action?loc=/pub/84f0210x/84f0210x2008000-eng.pdf&archive=1 (2011).
Brinsden, P. R. Controlling the high order multiple birth rate: the European perspective. Reprod. Biomed. Online 6, 339–344 (2003).
Reynolds, M. A., Schieve, L. A., Martin, J. A., Jeng, G. & Macaluso, M. Trends in multiple births conceived using assisted reproductive technology, United States, 1997–2000. Pediatrics 111, 1159–1162 (2003).
Wilcox, L. S., Kiely, J. L., Melvin, C. L. & Martin, M. C. Assisted reproductive technologies: estimates of their contribution to multiple births and newborn hospital days in the United States. Fertil. Steril. 65, 361–366 (1996).
Ananth, C. V., Joseph, K. S., Demissie, K. & Vintzileos, A. M. Trends in twin preterm birth subtypes in the United States, 1989 through 2000: impact on perinatal mortality. Am. J. Obstet. Gynecol. 193, 1076–1082 (2005).
Public Health Agency of Canada. Canadian Perinatal Health Report. (2008). https://www.canada.ca/en/public-health/services/reports-publications/canadian-perinatal-health-report-2008-edition.html. Accessed Feb 2018.
Alexander, G. R., Slay Wingate, M., Salihu, H. & Kirby, R. S. Fetal and neonatal mortality risks of multiple births. Obstet. Gynecol. Clin. North Am. 32, 1–16 (2005).
Rand, L., Eddleman, K. A. & Stone, J. Long-term outcomes in multiple gestations. Clin. Perinatol. 32, 495–513 (2005).
Pharoah, P. O. D. Neurological outcome in twins. Semin. Neonatol. 7, 223–230 (2002).
Nelson, K. B. & Ellenberg, J. H. Childhood neurological disorders in twins. Paediatr. Perinat. Epidemiol. 9, 135–145 (1995).
Vergani, P. et al. Predictors of adverse perinatal outcome in twins delivered at < 37 weeks. J. Matern. Fetal Neonatal Med. 16, 343–347 (2004).
Chau, V. et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann. Neurol. 66, 155–164 (2009).
Miller, S. P. et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J. Pediatr. 147, 609–616 (2005).
Guo, T. et al. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurology 88, 614–622 (2017).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Spittle, A., Orton, J., Anderson, P. J., Boyd, R. & Doyle, L. W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. CD005495 (2015).
Asztalos, E. V. et al. Association between primary caregiver education and cognitive and language development of preterm neonates. Am. J. Perinatol. 34, 364–371 (2017).
Beauregard, J. L., Drews-Botsch, C., Sales, J. M., Flanders, W. D. & Kramer, M. R. Preterm birth, poverty, and cognitive development. Pediatrics 141, e20170509 (2018).
Treyvaud, K. et al. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics 123, 555–561 (2009).
Benavente-Fernández, I. et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw. Open 2, e192914 (2019).
Woodward, L. J., Clark, C. A. C., Bora, S. & Inder, T. E. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS ONE 7, e51879 (2012).
Iwata, S. et al. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics 129, e1138–e1147 (2012).
Acknowledgements
We thank the staff of the Neonatal Follow-up Program at BC Women’s Hospital; Gisela Gosse research nurse for clinical chart review; and Ivan Cepeda, Cecil Chau, and Mary Beckingham for data collection. We appreciate the dedication of the families who participated with their infants at multiple visits in this longitudinal study. This study was supported by the Canadian Institutes of Health Research and Kids Brain Health Network.
Author information
Authors and Affiliations
Contributions
R.C.—analysis and interpretation of data, drafting the article and revising it critically, and final approval of the version to be published. V.C., A.S., R.E.G., and S.P.M.—conception and design, revising manuscript critically for important intellectual content, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all parents of the study participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Christensen, R., Chau, V., Synnes, A. et al. Longitudinal neurodevelopmental outcomes in preterm twins. Pediatr Res 90, 593–599 (2021). https://doi.org/10.1038/s41390-020-0840-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0840-7
This article is cited by
-
Chorionicity and neurodevelopmental outcomes in twin pregnancy: a systematic review and meta-analysis
Journal of Perinatology (2023)