Abstract
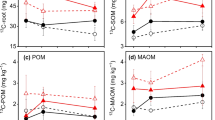
The mechanism that causes the difference in carbon (C) turnover rate in root populations is unclear. The carbon utilization strategy is assumed to be the main causal factor responsible for differences in root turnover rate. In this study, we determined the correlations between root turnover rate, production, and proportions of C allocated to roots using 13CO2 as a labeling gas in a 13C pulse labeling experiment. The proportions of δ13C were measured in various organs of the grass Bothriochloa ischaemum sampled 0, 6, 24, 48, 216, and 360 h after labeling in three treatments: control (CK), mild water stress (MS), and serious water stress (SS). We found that drought stress increased short-term C allocation to belowground. Fine roots have stronger C demand than coarse root under drought condition. The amount of 13C gradually decreased in leaves and increased in soil with time after 13C pulse labeling. Stem 13C increased with the level of stress and peaked at 24 h, while both fine- and coarse-root 13C peaked at 216 h. 13C distributed to fine roots in MS was significantly higher than in the other treatments at 216 h. The fine-root turnover rate in SS treatment was positively correlated with root biomass but not the amount of 13C. Larger C allocation to roots increased fine-root mass in MS, stimulated rapid fine-root turnover, and increased C input to both the rhizosphere and soil. The fine-root turnover in CK was significantly positively correlated with both 13C amount and biomass, which indicated that increasing short-term C input accelerated turnover in the fine-root pool. The C allocation difference between the fine roots and coarse roots may be a key cause of the different turnover rate in the root population.




Similar content being viewed by others
References
Bader M, Hiltbrunner E, Körner C (2009) Fine root responses of mature deciduous forest trees to free air carbon dioxide enrichment (FACE). Funct Ecol 23(5):913–921
Bahn M, Lattanzi FA, Hasibeder R, Wild B, Koranda M, Danese V, Brüggemann N, Schmitt M, Siegwolf R, Richter A (2013) Responses of belowground carbon allocation dynamics to extended shading in mountain grassland. New Phytol 198(1):116–126
Brunner I, Ostonen I (2013) Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362(1–2):357–372
Burton AJ, Pregitzer KS, Hendrick RL (2000) Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 125(3):389–399
Carbone M, Trumbore SE (2007) Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns. New Phytol 176(1):124–135
Carrillo Y, Dijkstra FA, Lecain D, Morgan JA, Blumenthal D, Waldron S, Pendall E (2014) Disentangling root responses to climate change in a semiarid grassland. Oecologia 175(2):699
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339(6127):1615–1618
Comas L, Bouma T, Eissenstat D (2002) Linking root traits to potential growth rate in six temperate tree species. Oecologia 132(1):34–43
Dirk G, Dietrich H, Christoph L (2009) Estimating fine root longevity in a temperate Norway spruce forest using three independent methods. Funct Plant Biol Fpb 36(1):11–19
Fahey TJ, Jacobs KR, Sherman RE (2012) Fine root turnover in sugar maple estimated by 13C isotope labeling. Can J For Res 42(10):1792–1795
Farrar JF, Jones DL (2000) The control of carbon acquisition by roots. New Phytol 147(1):43–53
Finér L, Ohashi M, Noguchi K, Hirano Y (2011) Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For Ecol Manage 262(11):2008–2023
Gaudinski J, Torn M, Riley W, Swanston C, Trumbore S, Joslin JD (2010a) Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Glob Change Biol 15(4):992–1014
Gaudinski JB, Torn MS, Riley WJ, Dawson TE, Joslin JD, Majdi H (2010b) Measuring and modeling the spectrum of fine-root turnover times in three forests using isotopes, minirhizotrons, and the Radix model. Global Biogeochem Cycles. https://doi.org/10.1029/2009GB003649
Gaudinski JB, Torn MS, Riley WJ, Swanston C, Trumbore SE, Joslin JD, Majdi H, Dawson TE, Hanson PJ (2009) Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Glob Change Biol 15(4):992–1014
Guo D, Mitchell RJ, Han W, Hendricks JJ, Fahey TJ, Hendrick RL (2008) Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol 177(2):443–456
Hansson K, Helmisaari HS, Sah SP, Lange H (2013) Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For Ecol Manage 309(12):58–65
Holdaway RJ, Richardson SJ, Dickie IA, Peltzer DA, Coomes DA (2011) Species- and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J Ecol 99(4):954–963
Johnson MG, Phillips DL, Tingey DT, Storm MJ (2000) Effects of elevated CO2, N-fertilization, and season on survival of ponderosa pine fine roots. Can J For Res 30(2):220–228
Keel SG, Campbell CD, Högberg MN, Richter A, Wild B, Zhou X, Hurry V, Linder S, Näsholm T, Högberg P (2012) Allocation of carbon to fine root compounds and their residence times in a boreal forest depend on root size class and season. New Phytol 194(4):972–981
Kollmann J, Dietz H, Edwards PJ (2004) Allocation, plasticity and allometry. Perspectives in Plant Ecology Evolution & Systematics 6(4):205–206
Langley J, Drake B, Hungate B (2002) Extensive belowground carbon storage supports roots and mycorrhizae in regenerating scrub oaks. Oecologia 131(4):542–548
Lee ET, Wang JW (2003) Statistical methods for survival data analysis, 3rd edn. Wiley, New York, pp 64–77
Leppälammi-Kujansuu J, Salemaa M, Dan BK, Linder S, Helmisaari HS (2014) Fine root turnover and litter production of Norway spruce in a long-term temperature and nutrient manipulation experiment. Plant Soil 374(1–2):73–88
Liu Y, Li P, Wang T, Liu Q, Wang W (2020) Root respiration and belowground carbon allocation respond to drought stress in a perennial grass (Bothriochloa ischaemum). CATENA 188:104449
Liu Y, Li P, Xu GC, Xiao L, Ren ZP, Li ZB (2017) Growth, morphological, and physiological responses to drought stress in Bothriochloa ischaemum. Frontiers in Plant Science 8:230
Liu Y, Wang G, Yu K, Li P, Xiao L, Liu G (2018) A new method to optimize root order classification based on the diameter interval of fine root. Scientific Reports 8(1):2960
Luo Y (2010) Uncertainties in interpretation of isotope signals for estimation of fine root longevity: theoretical considerations. Glob Change Biol 9(7):1118–1129
Lynch DJ, Matamala R, Iversen CM, Norby RJ, Gonzalez-Meler MA (2013) Stored carbon partly fuels fine-root respiration but is not used for production of new fine roots. New Phytol 199(2):420–430
Matamala R, Gonzàlezmeler MA, Jastrow JD, Norby RJ, Schlesinger WH (2003) Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 302(5649):1385–1387
Matamala R, Schlesinger WH (2010) Effects of elevated atmospheric CO2 on fine root production and activity in an intact temperate forest ecosystem. Glob Change Biol 6(8):967–979
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207(3):505–518
Metcalfe DB, Meir P, Williams M (2007) A comparison of methods for converting rhizotron root length measurements into estimates of root mass production per unit ground area. Plant Soil 301(1/2):279–288
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol. https://doi.org/10.1111/gcb.13850
Phillips RP, Meier IC, Bernhardt ES, Grandy AS, Wickings K, Finzi AC (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15(9):1042–1049
Poorter H, Karl JN, Peter BR, Oleksyn J, Poot P, Mommer L (2011) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193(1):30–50
Pregitzer KS, Deforest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine North American trees. Ecol Monogr 72(2):293–309
Pritchard S, Strand A, Mccormack M, Davis M, Finz A, Jackson R, Matamala R, Hh O, R. (2010) Fine root dynamics in a loblolly pine forest are influenced by free-air-CO2-enrichment: a six-year-minirhizotron study. Glob Change Biol 14(3):588–602
Reid JB, Crush JR (2013) Root turnover in pasture species: perennial ryegrass (Lolium perenne L). Crop Pasture Sci 64(2):165
Reid JB, Gray RAJ, Springett JA, Crush J (2015) Root turnover in pasture species: chicory, lucerne, perennial ryegrass and white clover. Annals of Applied Biology. https://doi.org/10.1111/aab.12228
Riley WJ, Gaudinski JB, Torn MS, Joslin JD, Hanson PJ (2010) Fine-root mortality rates in a temperate forest: estimates using radiocarbon data and numerical modeling. New Phytol 184(2):387–398
Sah SP, Jungner H, Oinonen M, Kukkola M, Helmisaari HS (2011) Does the age of fine root carbon indicate the age of fine roots in boreal forests? Biogeochemistry 104(1/3):91–102
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögelknabner I, Lehmann J, Manning DA (2011) Persistence of soil organic matter as an ecosystem property. Nature 478(7367):49–56
Solly E, Brunner I, Helmisaari H-S, Herzog C, Leppälammi-Kujansuu J, Schöning I, Schrumpf M, Schweingruber F, Trumbore S, Hagedorn F (2018) Unravelling the age of fine roots of temperate and boreal forests. Nature Communications 9(1):3006
Subke JA, Vallack HW, Leronni V, Baxter R, Ineson P (2012) Fast assimilate turnover revealed by in situ CO pulse-labelling in Subarctic tundra. Polar Biol 35(8):1209–1219
Tefs C, Gleixner G (2012) Importance of root derived carbon for soil organic matter storage in a temperate old-growth beech forest—evidence from C, N and 14 C content. For Ecol Manage 263:131–137
Trueman RJ, Gonzalezmeler MA (2010) Accelerated belowground C cycling in a managed agriforest ecosystem exposed to elevated carbon dioxide concentrations. Glob Change Biol 11(8):1258–1271
Trumbore SE, Davidson EA, Cook AC, Markewitz D, Richter DD (2001) The age of fine-root carbon in three forests of the Eastern United States measured by radiocarbon. Oecologia 129(3):420–429
Wang G, Liu F (2014) Carbon allocation of Chinese pine seedlings along a nitrogen addition gradient. For Ecol Manage 334:114–121
Wang G, Xue S, Liu F, Liu G (2017) Nitrogen addition increases the production and turnover of the lower-order roots but not of the higher-order roots of Bothriochloa ischaemum. Plant Soil 415(1–2):423–434
Watson CA, Ross JM, Bagnaresi U, Minotta GF, Roffi F, Atkinson D, Black KE, Hooker JE (2000) Environment-induced modifications to root longevity in Lolium perenne and Trifolium repens. Ann Bot 85(3):397–401
Wei L, Zhang X, Hou Z, Xu D, Yu X (2005) Effects of water stress on photosynthesis and carbon allocation in cunninghamia lanceolata seedlings. Acta Phytoecol Sin 29(3):394–402
Wells CE, Glenn DM, Eissenstat DM (2002) Changes in the risk of fine-root mortality with age: a case study in peach, Prunus persica (Rosaceae). Am J Bot 89(1):79–87
Yuan ZY, Chen HYH (2012) Indirect methods produce higher estimates of fine root production and turnover rates than direct methods. PLoS ONE 7(11):e48989
Acknowledgements
This research was funded by National key research and development program (Nos. 2016YFC0402407, 2016YFC0402404, and 2016ZZKT-13), National Natural Science Foundation of China (No. 41561144011), and the School foundation of Xi’an University of Technology (310–252071506).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, P., Xiao, L. et al. High Heterogeneity of Root Carbon Allocation Affects Root Turnover Rate and Production of Bothriochloa ischaemum Under Drought Stress. J Plant Growth Regul 40, 226–239 (2021). https://doi.org/10.1007/s00344-020-10090-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10090-8