Abstract
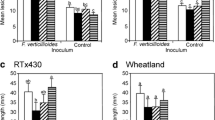
Ferulate 5-hydroxylase (F5H) of the monolignol pathway catalyzes the hydroxylation of coniferyl alcohol, coniferaldehyde and ferulic acid to produce 5-hydroxyconiferyl moieties, which lead to the formation of sinapic acid and syringyl (S) lignin monomers. In contrast, guaiacyl (G) lignin, the other major type of lignin monomer, is derived from polymerization of coniferyl alcohol. In this study, the effects of manipulating S-lignin biosynthesis in sorghum (Sorghum bicolor) were evaluated. Overexpression of sorghum F5H (SbF5H), under the control of the CaMV 35S promoter, increased both S-lignin levels and the ratio of S/G lignin, while plant growth and development remained relatively unaffected. Maüle staining of stalk and leaf midrib sections from SbF5H overexpression lines indicated that the lignin composition was altered. Ectopic expression of SbF5H did not affect the gene expression of other monolignol pathway genes. In addition, brown midrib 12-ref (bmr12-ref), a nonsense mutation in the sorghum caffeic acid O-methyltransferase (COMT) was combined with 35S::SbF5H through cross-pollination to examine effects on lignin synthesis. The stover composition from bmr12 35S::SbF5H plants more closely resembled bmr12 stover than 35S::SbF5H or wild-type (WT) stover; S-lignin and total lignin concentrations were decreased relative to WT or 35S::SbF5H. Likewise, expression of upstream monolignol biosynthetic genes was increased in both bmr12 and bmr12 35S::SbF5H relative to WT or 35S::SbF5H. Overall, these results indicated that overexpression of SbF5H did not compensate for the loss of COMT activity.
Key message
Overexpression of F5H in sorghum increases S-lignin without increasing total lignin content or affecting plant growth, but it cannot compensate for the loss of COMT activity in monolignol synthesis.









Similar content being viewed by others
References
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bout S, Vermerris W (2003) A candidate-gene approach to clone the sorghum Brown midrib gene encoding caffeic acid O-methyltransferase. Mol Genet Genomics 269:205–214
Chapple C, Vogt T, Ellis BE, Somerville CR (1992) An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell 4(11):1413–1424
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761
Cherney J, Cherney D, Akin D, Axtell J (1991) Potential of brown-midrib, low-lignin mutants for improving forage quality. In: Advances in agronomy. Elsevier, Amsterdam, pp 157–198
Crocker EC (1933) Maule lignin test on podocarpus wood. Bot Gaz 95:168–171
Dien BS, Sarath G, Pedersen JF, Sattler SE, Chen H, Funnell-Harris DL, Nichols NN, Cotta MA (2009) Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. Bioenergy Res 2:153–164
Eudes A, Dutta T, Deng K, Jacquet N, Sinha A, Benites VT, Baidoo EEK, Richel A, Sattler SE, Northen TR, Singh S, Simmons BA, Loque D (2017) SbCOMT (Bmr12) is involved in the biosynthesis of tricin-lignin in sorghum. PLoS ONE 12:e0178160
Franke R, Mcmichael CM, Meyer K, Shirley AM, Cusumano JC, Chapple C (2000) Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. Plant J 22:223–234
Godin B, Nagle N, Sattler S, Agneessens R, Delcarte J, Wolfrum E (2016) Improved sugar yields from biomass sorghum feedstocks: comparing low-lignin mutants and pretreatment chemistries. Biotechnol Biofuels 9:251
Guo D, Chen F, Wheeler J, Winder J, Selman S, Peterson M, Dixon RA (2001) Improvement of in-rumen digestibility of alfalfa forage by genetic manipulation of lignin O-methyltransferases. Transgenic Res 10:457–464
Hatfield RD, Rancour DM, Marita JM (2017) Grass cell walls: a story of cross-linking. Front Plant Sci 7:2056
Hill-Ambroz KL, Weeks JT (2001) Comparison of constitutive promoters for sorghum [Sorghum bicolor (L.) Moench] transformation. Cereal Res Commun 29:17–24
Humphreys JM, Hemm MR, Chapple C (1999) New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci USA 96:10045–10050
Huntley SK, Ellis D, Gilbert M, Chapple C, Mansfield SD (2003) Significant increases in pulping efficiency in C4H–F5H-transformed poplars: improved chemical savings and reduced environmental toxins. J Agric Food Chem 51:6178–6183
Jorgenson LR (1931) Brown midrib in maize and its linkage relations. J Am Soc Agron 23:549–557
Jouanin L, Goujon T, De Nadaï V, Martin M-T, Mila I, Vallet C, Pollet B, Yoshinaga A, Chabbert B, Petit-Conil M, Lapierre C (2000) Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol 123:1363–1374
Jung HG, Deetz DA (1993) Cell wall lignification and degradability. In: Jung HG, Buxton DR, Hatfield RD, Ralph J (eds) Forage cell wall structure and digestibility. American Society of Agronomy Crop Science Society of America, Soil Science Society of America, Madison, pp 315–346
Lee DK, Aberle E, Anderson EK, Anderson W, Baldwin BS, Baltensperger D, Barrett M, Blumenthal J, Bonos S, Bouton J, Bransby DI, Brummer C, Burks PS, Chen C, Daly C, Egenolf J, Farris RL, Fike JH, Gaussoin R, Gill JR, Gravois K, Halbleib MD, Hale A, Hanna W, Harmoney K, Heaton EA, Heiniger RW, Hoffman L, Hong CO, Kakani G, Kallenbach R, Macoon B, Medley JC, Missaoui A, Mitchell R, Moore KJ, Morrison JI, Odvody GN, Richwine JD, Ogoshi R, Parrish JR, Quinn L, Richard E, Rooney WL, Rushing JB, Schnell R, Sousek M, Staggenborg SA, Tew T, Uehara G, Viands DR, Voigt T, Williams D, Williams L, Wilson LT, Wycislo A, Yang Y, Owens V (2018) Biomass production of herbaceous energy crops in the United States: field trial results and yield potential maps from the multiyear regional feedstock partnership. GCB Bioenergy 10:698–716
Li M, Pu Y, Ragauskas AJ (2016) Current understanding of the correlation of lignin structure with biomass recalcitrance. Front Chem 4:45
Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple C (1998) Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA 95:6619–6623
Mitchell R, Schmer M, Anderson W, Jin V, Balkcom K, Kiniry J, Coffin A, White P (2016) Dedicated energy crops and crop residues for bioenergy feedstocks in the central and eastern USA. Bioenergy Res 9:384–398
Odell JT, Nagy F, Chua N-H (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313:810–812
Oliver A, Pedersen J, Grant R, Klopfenstein T (2005) Comparative effects of the sorghum bmr-6 and bmr-12 genes: I. Forage sorghum yield and quality. Crop Sci 45:2234–2239
Palmer NA, Sattler SE, Saathoff AJ, Funnell D, Pedersen JF, Sarath G (2008) Genetic background impacts soluble and cell wall-bound aromatics in brown midrib mutants of sorghum. Planta 229:115
Pedersen JF, Funnell DL, Toy JJ, Oliver A, Grant R (2006) Registration of twelve grain sorghum genetic stocks near-isogenic for the brown midrib genes bmr-6 and bmr-12. Crop Sci 46:491–493
Piquemal J, Chamayou S, Nadaud I, Beckert M, Barrière Y, Mila I, Lapierre C, Rigau J, Puigdomenech P, Jauneau A, Digonnet C, Boudet A-M, Goffner D, Pichon M (2002) Down-regulation of caffeic acid O-methyltransferase in maize revisited using a transgenic approach. Plant Physiol 130:1675–1685
Porter KS, Anxtell JD, Lechtenberg VL, Colenbrander VF (1978) Phenotype, fiber composition, and in vitro dry matter disappearance of chemically induced brown midrib (bmr) mutants of sorghum. Crop Sci. https://doi.org/10.2135/cropsci1978.0011183X001800020002x
Pradhan Mitra P, Loqué D (2014) Histochemical staining of Arabidopsis thaliana secondary cell wall elements. J Vis Exp. https://doi.org/10.3791/51381
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843
Ralph J, Lapierre C, Marita JM, Kim H, Lu F, Hatfield RD, Ralph S, Chapple C, Franke R, Hemm MR, Van Doorsselaere J, Sederoff RR, O’Malley DM, Scott JT, Mackay JJ, Yahiaoui N, Boudet A-M, Pean M, Pilate G, Jouanin L, Boerjan W (2001) Elucidation of new structures in lignins of CAD- and COMT-deficient plants by NMR. Phytochemistry 57:993–1003
Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev 3:29–60
Rastogi S, Dwivedi UN (2006) Down-regulation of lignin biosynthesis in transgenic Leucaena leucocephala harboring O-methyltransferase gene. Biotechnol Prog 22:609–616
Reddy MSS, Chen F, Shadle G, Jackson L, Aljoe H, Dixon RA (2005) Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc Natl Acad Sci USA 102:16573–16578
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Rooney WL (2004) Sorghum improvement—integrating traditional and new technology to produce improved genotypes. Adv Agron 83:37–109
Saballos A, Ejeta G, Sanchez E, Kang CH, Vermerris W (2009) A genomewide analysis of the cinnamyl alcohol dehydrogenase family in sorghum [Sorghum bicolor (L.) Moench] identifies SbCAD2 as the brown midrib6 gene. Genetics 181:783–795
Saballos A, Sattler SE, Sanchez E, Foster TP, Xin Z, Kang C, Pedersen JF, Vermerris W (2012) Brown midrib2 (Bmr2) encodes the major 4-coumarate:coenzyme A ligase involved in lignin biosynthesis in sorghum (Sorghum bicolor (L.) Moench). Plant J 70:818–830
Sarath G, Mitchell RB, Sattler SE, Funnell D, Pedersen JF, Graybosch RA, Vogel KP (2008) Opportunities and roadblocks in utilizing forages and small grains for liquid fuels. J Ind Microbiol Biotechnol 35:343–354
Sattler SE, Saathoff AJ, Haas EJ, Palmer NA, Funnell-Harris DL, Sarath G, Pedersen JF (2009) A nonsense mutation in a cinnamyl alcohol dehydrogenase gene is responsible for the sorghum brown midrib6 phenotype. Plant Physiol 150:584–595
Sattler SE, Funnell-Harris DL, Pedersen JF (2010) Efficacy of singular and stacked brown midrib 6 and 12 in the modification of lignocellulose and grain chemistry. J Agric Food Chem 58:3611–3616
Sattler SE, Palmer NA, Saballos A, Greene AM, Xin Z, Sarath G, Vermerris W, Pedersen JF (2012) Identification and characterization of four missense mutations in brown midrib 12 (Bmr12), the caffeic O-methyltransferase (COMT) of sorghum. Bioenergy Res 5:855–865
Scully ED, Gries T, Sarath G, Palmer NA, Baird L, Serapiglia MJ, Dien BS, Boateng AA, Ge Z, Funnell-Harris DL, Twigg P, Clemente TE, Sattler SE (2016) Overexpression of SbMyb60 impacts phenylpropanoid biosynthesis and alters secondary cell wall composition in Sorghum bicolor. Plant J 85:378–395
Sewalt VJ, Ni W, Blount JW, Jung HG, Masoud SA, Howles PA, Lamb C, Dixon RA (1997) Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of l-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol 115:41–50
Stewart JJ, Akiyama T, Chapple C, Ralph J, Mansfield SD (2009) The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol 150:621–635
Takeda Y, Koshiba T, Tobimatsu Y, Suzuki S, Murakami S, Yamamura M, Rahman MM, Takano T, Hattori T, Sakamoto M (2017) Regulation of CONIFERALDEHYDE 5-HYDROXYLASE expression to modulate cell wall lignin structure in rice. Planta 246:337–349
Tetreault HM, Scully ED, Gries T, Palmer NA, Funnell-Harris DL, Baird L, Seravalli J, Dien BS, Sarath G, Clemente TE, Sattler SE (2018) Overexpression of the Sorghum bicolor SbCCoAOMT alters cell wall associated hydroxycinnamoyl groups. PLoS ONE 13:e0204153
Towers GHN, Gibbs RD (1953) Lignin chemistry and the taxonomy of higher plants. Nature 172:25–26
Vanholme R, Storme V, Vanholme B, Sundin L, Christensen JH, Goeminne G, Halpin C, Rohde A, Morreel K, Boerjan W (2012) A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell. https://doi.org/10.1105/tpc.112.102574
Vermerris W, Thompson KJ, Mcintyre LM, Axtell JD (2002) Evidence for an evolutionarily conserved interaction between cell wall biosynthesis and flowering in maize and sorghum. BMC Evol Biol 2:2
Vignols F, Rigau J, Torres MA, Capellades M, Puigdomenech P (1995) The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell 7:407–416
Vogel KP, Pedersen JF, Masterson SD, Toy JJ (1999) Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop Sci 39:276–279
Weng JK, Mo H, Chapple C (2010) Over-expression of F5H in COMT-deficient Arabidopsis leads to enrichment of an unusual lignin and disruption of pollen wall formation. Plant J 64:898–911
Wu Z, Wang N, Hisano H, Cao Y, Wu F, Liu W, Bao Y, Wang Z-Y, Fu C (2019) Simultaneous regulation of F5H in COMT-RNAi transgenic switchgrass alters effects of COMT suppression on syringyl lignin biosynthesis. Plant Biotechnol J 17:836–845
Yue F, Lu F, Sun R-C, Ralph J (2012) Syntheses of lignin-derived thioacidolysis monomers and their uses as quantitation standards. J Agric Food Chem 60:922–928
Yue F, Lu F, Regner M, Sun R, Ralph J (2017) Lignin-derived thioacidolysis dimers: reevaluation, new products, authentication, and quantification. Chemsuschem 10:830–835
Zhao H, Wei J, Jinyu Z, Liu H, Tai W, Song Y (2002) Lignin biosynthesis by suppression of two O-methyl-transferases. Chin Sci Bull 47:1092–1095
Acknowledgements
We thank John Toy, Coehn Preble, Samantha Timmons and Sara Finnegan for technical assistance, and Dr. Heather Van Buskirk for critically reviewing the manuscript. We thank the Plant Transformation Core Research Facility at the University of Nebraska for creating the overexpression lines. This research was supported by the United States Department of Agriculture: National Institute of Food and Agriculture AFRI Grant Number 2011-67009-30026 (SES and DLF-H) and additional funding from USDA-ARS, CRIS Projects 3042-21220-033-00-D (SES and DLF-H). The University of Nebraska DNA Sequencing Core receives partial support from the National Institute for General Medical Science (NIGMS) INBRE-P20GM103427-14 and COBRE-1P30GM110768-01 Grants as well as The Fred and Pamela Buffett Cancer Center Support Grant: P30CA036727. This publication’s contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or NIGMS. The US Department of Agriculture, Agricultural Research Service, is an equal opportunity/affirmative action employer and all agency services are available without discrimination. Mention of commercial products and organizations in this manuscript is solely to provide specific information. It does not constitute endorsement by USDA-ARS over other products and organizations not mentioned.
Author information
Authors and Affiliations
Contributions
HMT, SES and DLFH designed the research; HMT, TG, NAP, SS and ZG performed the experiments; HMT, TG, NAP, DLFH, GS and SES analyzed and interpreted the data; HMT and SES wrote the first draft of the manuscript, and all authors reviewed and revised the manuscript prior to publication.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tetreault, H.M., Gries, T., Palmer, N.A. et al. Overexpression of ferulate 5-hydroxylase increases syringyl units in Sorghum bicolor. Plant Mol Biol 103, 269–285 (2020). https://doi.org/10.1007/s11103-020-00991-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-020-00991-3