Abstract
Introduction
Glioblastoma multiforme (GBM) is one of the most devastating brain malignancies worldwide and is considered to be incurable. However, the mechanisms underlying its aggressiveness remain unclear.
Methods
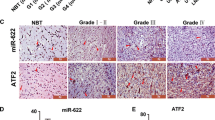
The expression of ADAM17 in tissue samples was detected by immunohistochemistry. Knockdown and rescue experiments were used to demonstrate the regulatory effect of ADAM17 on the invasion ability of GBM cells. Western Blot and qPCR were used to detect the expression of related proteins and RNAs. Moreover, a luciferase reporter assay was performed to verify whether miR-145 directly binds to the 3′-UTR of ADAM17.
Results
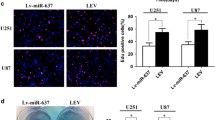
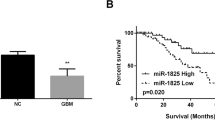
We revealed that ADAM17 was overexpressed in GBM tissues and correlated positively with poor prognosis. The knockdown of ADAM17 obviously suppressed the invasiveness of GBM cell lines. Furthermore, we found that knockdown of ADAM17 decreased activation of EGFR/Akt/C/EBP-β signaling, and consequently upregulated miR-145 expression in GBM cell lines. Notably, miR-145 directly targeted the ADAM17 3′-UTR and suppressed expression levels of ADAM17.
Conclusions
Our findings define an ADAM17/EGFR/miR-145 feedback loop that drives the GBM invasion. Reciprocal regulation between ADAM17 and miR-145 results in aberrant activation of EGFR signaling, suggesting that inhibition of ADAM17 expression can be an ideal therapeutic strategy for the treatment of GBM.






Similar content being viewed by others
References
Van Meir EG, Hadjipanayis CG, Norden AD et al (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60:166–193. https://doi.org/10.3322/caac.20069
Lapointe S, Perry A, Butowski NA (2018) Primary brain tumours in adults. Lancet 392:432–446. https://doi.org/10.1016/S0140-6736(18)30990-5
Paw I, Carpenter RC, Watabe K et al (2015) Mechanisms regulating glioma invasion. Cancer Lett 362:1–7. https://doi.org/10.1016/j.canlet.2015.03.015
Herrlich P, Herrlich A (2017) ADAM metalloprotease-released cancer biomarkers. Trends Cancer 3:482–490. https://doi.org/10.1016/j.trecan.2017.05.001
Mullooly M, McGowan PM, Crown J, Duffy MJ (2016) The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol Ther 17:870–880. https://doi.org/10.1080/15384047.2016.1177684
Rossello A, Nuti E, Ferrini S, Fabbi M (2016) Targeting ADAM17 sheddase activity in cancer. Curr Drug Targets 17:1908–1927. https://doi.org/10.2174/1389450117666160727143618
Moss ML, Minond D (2017) Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediators Inflamm 2017:1–21. https://doi.org/10.1155/2017/9673537
McGowan PM, Ryan BM, Hill ADK et al (2007) ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin Cancer Res 13:2335–2343. https://doi.org/10.1158/1078-0432.CCR-06-2092
Wu B, Sha L, Wang Y et al (2014) Diagnostic and prognostic value of a disintegrin and metalloproteinase-17 in patients with gliomas. Oncol Lett 8:2616–2620. https://doi.org/10.3892/ol.2014.2582
Aydin D, Bilici A, Yavuzer D et al (2015) Prognostic significance of ADAM17 expression in patients with gastric cancer who underwent curative gastrectomy. Clin Transl Oncol 17:604–611. https://doi.org/10.1007/s12094-015-1283-1
Sun J, Li D-M, Huang J et al (2017) The correlation between the expression of ADAM17, EGFR and Ki-67 in malignant gliomas. Eur Rev Med Pharmacol Sci 21:4595–4599
Bartel DP (2004) MicroRNAs. Cell 116:281–297. https://doi.org/10.1016/S0092-8674(04)00045-5
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16:203–222. https://doi.org/10.1038/nrd.2016.246
Luo JW, Wang X, Yang Y, Mao Q (2015) Role of micro-RNA (miRNA) in pathogenesis of glioblastoma. Eur Rev Med Pharmacol Sci 19:1630–1639
Michael MZ, O’Connor SM, van Holst Pellekaan NG et al (2003) Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res (MCR) 1:882–891
Cho WCS, Chow ASC, Au JSK (2011) MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol 8:125–131. https://doi.org/10.4161/rna.8.1.14259
Lei C, Du F, Sun L et al (2017) miR-143 and miR-145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6. Cell Death Dis 8:e3101–e3101. https://doi.org/10.1038/cddis.2017.493
Sachdeva M, Mo Y-Y (2010) MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Can Res 70:378–387. https://doi.org/10.1158/0008-5472.CAN-09-2021
Qiu T, Zhou X, Wang J et al (2014) MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett 588:1168–1177. https://doi.org/10.1016/j.febslet.2014.02.054
Cui S-Y, Wang R, Chen L-B (2014) MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med 18:1913–1926. https://doi.org/10.1111/jcmm.12358
Zhu H, Dougherty U, Robinson V et al (2011) EGFR signals downregulate tumor suppressors miR-143 and miR-145 in western diet-promoted murine colon cancer: role of G1 regulators. Mol Cancer Res 9:960–975. https://doi.org/10.1158/1541-7786.MCR-10-0531
Koo S, Martin G, Toussaint LG (2015) MicroRNA-145 promotes the phenotype of human glioblastoma cells selected for invasion. Anticancer Res 35:3209–3215
Kaller M, Hermeking H (2016) Interplay between transcription factors and microRNAs regulating epithelial-mesenchymal transitions in colorectal cancer. Adv Exp Med Biol 937:71–92
Bracken CP, Scott HS, Goodall GJ (2016) A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 17:719–732. https://doi.org/10.1038/nrg.2016.134
Hassemer EL, Endres B, Toonen JA et al (2013) ADAM17 transactivates EGFR signaling during embryonic eyelid closure. Investig Opthalmol Vis Sci 54:132. https://doi.org/10.1167/iovs.12-11130
Sachdeva M, Liu Q, Cao J et al (2012) Negative regulation of miR-145 by C/EBP-β through the Akt pathway in cancer cells. Nucleic Acids Res 40:6683–6692. https://doi.org/10.1093/nar/gks324
McGowan PM, Mullooly M, Caiazza F et al (2013) ADAM-17: a novel therapeutic target for triple negative breast cancer. Ann Oncol 24:362–369. https://doi.org/10.1093/annonc/mds279
Fang W, Qian J, Wu Q et al (2017) ADAM-17 expression is enhanced by FoxM1 and is a poor prognostic sign in gastric carcinoma. J Surg Res 220:223–233. https://doi.org/10.1016/j.jss.2017.06.032
Baumgart A, Seidl S, Vlachou P et al (2010) ADAM17 regulates epidermal growth factor receptor expression through the activation of notch1 in non-small cell lung cancer. Can Res 70:5368–5378. https://doi.org/10.1158/0008-5472.CAN-09-3763
Mustafi R, Dougherty U, Mustafi D et al (2017) ADAM17 is a Tumor promoter and therapeutic target in western diet-associated colon cancer. Clin Cancer Res 23:549–561. https://doi.org/10.1158/1078-0432.CCR-15-3140
Chen X, Chen L, Chen J et al (2013) ADAM17 promotes U87 glioblastoma stem cell migration and invasion. Brain Res 1538:151–158. https://doi.org/10.1016/j.brainres.2013.02.025
Chen X, Chen L, Zhang R et al (2013) ADAM17 regulates self-renewal and differentiation of U87 glioblastoma stem cells. Neurosci Lett 537:44–49. https://doi.org/10.1016/j.neulet.2013.01.021
Wolpert F, Tritschler I, Steinle A et al (2014) A disintegrin and metalloproteinases 10 and 17 modulate the immunogenicity of glioblastoma-initiating cells. Neuro-Oncology 16:382–391. https://doi.org/10.1093/neuonc/not232
Zunke F, Rose-John S (2017) The shedding protease ADAM17: physiology and pathophysiology. Biochim Biophys Acta (BBA) 1864:2059–2070. https://doi.org/10.1016/j.bbamcr.2017.07.001
Miller MA, Oudin MJ, Sullivan RJ et al (2016) Reduced proteolytic shedding of receptor tyrosine kinases is a post-translational mechanism of kinase inhibitor resistance. Cancer Discov 6:382–399. https://doi.org/10.1158/2159-8290.CD-15-0933
Shimoda M, Horiuchi K, Sasaki A et al (2016) Epithelial cell-derived a disintegrin and metalloproteinase-17 confers resistance to colonic inflammation through EGFR activation. EBioMedicine 5:114–124. https://doi.org/10.1016/j.ebiom.2016.02.007
Zheng X, Jiang F, Katakowski M et al (2009) ADAM17 promotes breast cancer cell malignant phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther 8:1045–1054. https://doi.org/10.4161/cbt.8.11.8539
Meng X, Hu B, Hossain MM et al (2016) ADAM17-siRNA inhibits MCF-7 breast cancer through EGFR-PI3K-AKT activation. Int J Oncol. https://doi.org/10.3892/ijo.2016.3536
Berens ME, Rief MD, Shapiro JR et al (1996) Proliferation and motility responses of primary and recurrent gliomas related to changes in epidermal growth factor receptor expression. J Neurooncol 27:11–22. https://doi.org/10.1007/BF00146079
Lund-Johansen M, Bjerkvig R, Humphrey PA et al (1990) Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Can Res 50:6039–6044
Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 9:52. https://doi.org/10.3390/cancers9050052
Cai M, Wang Z, Zhang J et al (2015) Adam17, a target of Mir-326, promotes emt-induced cells invasion in lung adenocarcinoma. Cell Physiol Biochem 36:1175–1185. https://doi.org/10.1159/000430288
Su Y, Wang Y, Zhou H et al (2014) MicroRNA-152 targets ADAM17 to suppress NSCLC progression. FEBS Lett 588:1983–1988. https://doi.org/10.1016/j.febslet.2014.04.022
Lu Y, Chopp M, Zheng X et al (2013) MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol Rep 29:67–72. https://doi.org/10.3892/or.2012.2084
Wu J, Yin L, Jiang N et al (2015) MiR-145, a microRNA targeting ADAM17, inhibits the invasion and migration of nasopharyngeal carcinoma cells. Exp Cell Res 338:232–238. https://doi.org/10.1016/j.yexcr.2015.08.006
Lan T, Wang H, Zhang Z et al (2017) Downregulation of β-arrestin 1 suppresses glioblastoma cell malignant progression vis inhibition of Src signaling. Exp Cell Res 357:51–58. https://doi.org/10.1016/j.yexcr.2017.04.023
Chen J, Lan T, Zhang W et al (2015) Platelet-activating factor receptor-mediated PI3K/AKT activation contributes to the malignant development of esophageal squamous cell carcinoma. Oncogene 34:5114–5127. https://doi.org/10.1038/onc.2014.434
Doberstein K, Steinmeyer N, Hartmetz AK et al (2013) MicroRNA-145 targets the metalloprotease ADAM17 and is suppressed in renal cell carcinoma patients. Neoplasia (United States). https://doi.org/10.1593/neo.121222
Acknowledgements
This study was supported by the Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (No. KZ201510025030).
Author information
Authors and Affiliations
Contributions
Conception and design: Yuduo Guo, Xin He, Junfa Li, Chunjiang Yu, Hongwei Zhang. Development of methodology: Yuduo Guo, Xin He, Junfa Li, Chunjiang Yu, Hongwei Zhang. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Yuduo Guo, Xin He, Junfa Li, Chunjiang Yu, Hongwei Zhang. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Yuduo Guo, Xin He, Junfa Li, Chunjiang Yu, Hongwei Zhang. Writing, review, and/or revision of the manuscript: Yuduo Guo, Junfa Li, Chunjiang Yu, Hongwei Zhang. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Yuduo Guo, Xin He, Mingshan Zhang, Yanming Qu, Chunyu Gu, Ming Ren, Haoran Wang, Weihai Ning Study supervision: Junfa Li, Chunjiang Yu, Hongwei Zhang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Y., He, X., Zhang, M. et al. Reciprocal control of ADAM17/EGFR/Akt signaling and miR-145 drives GBM invasiveness. J Neurooncol 147, 327–337 (2020). https://doi.org/10.1007/s11060-020-03453-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03453-4