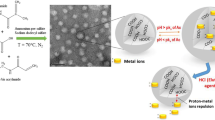
Abstract
Nanospheres with different particles size and nanorodes of copolymer of anthranilic acid with o-aminophenol poly(AA-co-o-AP) were synthesized by redox polymerization initiated by FeSO4.7H2O as redox initiator and ammonium peroxydisulfate (APS) as oxidant in different concentrations of aqueous solutions of hydrochloric acid. The influence of synthetic parameters such as acid concentration and the presence of redox initiator were investigated. The morphology and particles size were studied by transmission electron microscope (TEM) and scanning electron microscope (SEM). The results showed that the morphology and average particle size of polymeric nano particles according to SEM and TEM analyses were different based upon the conditions of the copolymerization. The physico-chemical characterization of the prepared nanoparticles was carried out by Fourier transform infrared spectroscopy (FT-IR), X-ray powder diffraction (XRD) and Thermogravimetric(TGA). Which FT-IR confirmed the structure of poly(AA-co-o-AP) nanoparticles in emeraldine form. The molecular weight was determined by gel permeation chromatography (GPC). The surface area of nanocopolymer particles was determined also by Brunauer-Emmett-Teller (BET). The competition of the prepared nano-sized copolymers particles towards the adsorption of copper ions from aqueous solutions was investigated. The results showed that the adsorption capacity was based on particle size of nanocopolymers and their surface area. The adsorption capacity increased with decreasing the particle size. On the other hand the adsorption capacity increased with increasing the surface area and the molecular weight of the prepared nano-sized copolymers of poly(AA-co-o-AP).






Similar content being viewed by others
References
Khalil AA, Shaaban AF, Azab MM, Mahmoud AA, Metwally AM (2013) Synthesis, characterization and morphology of polyanthranilic acid micro- and nanostructures. J. Polym. Res. 20:142–152
Mallakpour S, Behranvand V (2016) Polymeric nanoparticles: recent development in synthesis and application. Express Polym Lett 10:895–913
Mohanraj VJ, Chen Y (2006) Nanoparticles. Trop. J. Pharm. Res. 5:561–573
Mahalingam M, Krishnamoorthy K (2015) Selection of a suitable method for the preparation of polymeric nanoparticles. Adv Pharm Bull 5:57–67
Crucho CIC, Barros MT (2017) Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater. Sci. Eng. C 80:771–784
Hornig S, Heinze T, Becer CR, Schubert US (2009) Synthetic polymeric nanoparticles by nanoprecipitation. J. Mater. Chem. 19:3838–3840
Schmid G (2004) Nanoparticles: from theory to applications. Willey-VCH Publishers, Weinheim
Geckeler KE, Rosenberg E (eds) (2006) Functional nanomaterials. American Scientific Puplishers, Valencia
Hosokawa M, Nogi K, Yokoyama T (2007) Nanoparticle technology handbook. Elseiver, Amsterdam
Geckeler KE, Nishide H (eds) (2010) Advanced nanomaterials. Willey-VCH Publishers, Weinheim
Wang X, Summers CJ, Wang ZL (2004) Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 4:423–426
Jang JS, Oh JH (2002) Novel crystalline supramolecular assemblies of amorphous polypyrrole nanoparticles through surfactant templating. Chem. Commun. 19:2200–2201
Fudouzi H, Xia Y (2003) Photonic papers and inks: color writing with colorless materials. Adv. Mater. 15:892–896
Brahim S, Narinesingh D, Elie GA (2001) Amperometric determination of cholesterol in serum using a biosensor of cholesterol oxidase contained within a polypyrrole-hydrogel membrane. Anal. Chim. Acta 448:27–36
Zhang Q, Chuang KT (2001) Adsorption of organic pollutants from effluents of a Kraft pulp mill on activated carbon and polymer resin. Adv. Environ. Res. 5:251–258
Nagavarma BVN, Yadav HKS, Ayaz A, Vasudha LS, Shivakumar HG (2012) Different techniques for preparation of polymeric nanoparticles. Asian J Pharm Clin Res 5:16–23
Staff RH, Landfester K, Crespy D (2013) Recent advances in the emulsion solvent evaporation technique for the preparation of nanoparticles and nanocapsules. Adv. Polym. Sci. 262:329–344
Vauthier C, Bouchemal K (2009) Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 26:1025–1059
Zoromba MS, Abdel-Aziz MH, Bassyouni M, El-Ashtoukhy ESZ, Abdel-Hamid SMS (2019) Synthesis of o-aminophenol-m-phenylenediamine copolymer. J. Polym. Res. 26:34–42
Al-Hossainy AF, Zoromba MS (2019) Doped-poly (Para-nitroaniline- co aniline): synthesis, semiconductor characteristics, density, functional theory and photoelectric properties. J. Alloys Compd. 78:1–10
Zoromba MS, Ismail MIM, Bassyouni M, Abdel-Aziz MH, Salah N, Alshahrie A, Memic A (2017) Fabrication and characterization of poly (aniline-co-o- anthranilic acid)/ magnetite nanocomposites and their application in wastewater treatment. Colloid Surface A: Physicochem Eng Aspects 520:121–130
Zoromba MS, Al-Hossainy AF, Abdel-Aziz MH (2017) Conductive thin films based on poly (aniline-co-o-anthranilic acid)/ magnetite nanocomposite for photovoltaic applications. Synth. Met. 231:34–43
Chen CH, KoC J, Chuang CH, Mao CF, Liao WT, Hsieh CD (2017) Synthesis and characterization of polyaniline co-doped with nitric acid and dodecyl benzene sulfonic acid. J. Polym. Res. 24:10–20
Al-Hossainy AF, Bassyouni M, Zoromba MSh (2018) Elucidation of Electrical and Optical Parameters of Poly(o-anthranilic acid)-poly(o-amino phenol)/Copper Oxide Nanocomposites Thin Films. J Inorg. Organomet. Polym. Mater 28: 2572–2583.
Abdel-Aziz MH, Al-Hossainy AF, Ibrahim A, Abd El-Maksoud SA, Zoromba MS, Bassyouni M, Abdel-Hamid SMS, Abd-Elmageed AAI, Elsayed IA, Alqahtani OM (2018) Synthesis, characterization and optical properties of multiwalled carbon nanotubes/aniline-o-anthranilic acid copolymer nanocomposite thin films. J. Mater. Sci. 29:16702–16714
Thenmozhi G, Arockiasamy P, Santhi RJ (2014) Isomers of poly aminophenol: chemical synthesis, characterization, and its corrosion protection aspect on mild steel in 1M HCl. Int. J. Electrochem. Sci. 2014:1–12
Gu J, Kan S, Shen Q, Kan J (2014) Effects of Sulfanilic acid and Anthranilic acid on electrochemical stability of Polyaniline. Int. J. Electrochem. Sci. 9:6858–6869
Li D, Kaner RB (2006) Shape and aggregation control of nanoparticles:not shaken, not stirred. J. Am. Chem. Soc. 128:968–975
Li G, Zhang C, Li Y, Peng H, Chen K (2010) Rapid polymerization initiated by redox initiator for the synthesis of polyaniline nanofibres. Polymer 51:1934–1939
Odian G (2004) Principles of polymerization4th edn. Wiley, Hoboken, p 216
Tran HD, Wang Y, D’Arcy JM, Kaner RB (2008) Toward an understanding of the formation of conducting polymer nanofibers. ACS Nano 2:1841–1848
Ghonem MA, El-Enany G (2016) Development of coducing poly(o-amino phenol) film and its capacitance behaviour. Int. J. Electrochem. Sci. 11:9987–9997
Yakuphanoglu F, Basaran E, Senkal BF, Sezer E (2006) Electrical and optical properties of an organic semiconductor based on polyaniline prepared by emulsion polymerization and fabrication of Ag/polyaniline/n-SiSchottkydiode. J. Phys. Chem. B 110:16908–16913
Quillard S, Louarn G, Buisson JP, Boyer M, Lapkowski M, Pron A (1997) Vibrational spectroscopic studies of the isotope effects in polyaniline. Synth. Met. 84:805–806
Shaaban AF, Khalil AA, Radwan M, El-Hefnawy M, El-Khawaga HA (2017) Synthesis, characterization and application of a novel nanometer-sized chelating resin for removal of cu(II), co(II) and Ni(II) ions from aqueous solutions. J. Polym. Res. 62:24–165
Saravanan C, Palaniappan S, Chandezon S (2008) Synthesis of nanoporous conducting polyaniline using ternary surfactant. J Mat Lett 62:882–885
Dufour B, Rannou P, Fedorko P, Djurado D, Travers JP, Pron A (2001) Effect of plasticizing dopants on spectroscopic properties, Supramolecular structure, and electrical transport in metallic Polyaniline. Chem. Mater. 13:4032–4040
Xia H, Wang Q (2002) Ultrasonic irradiation: a novel approach to prepare conductive polyaniline/nanocrystalline titanium oxide composites. Chem. Mater. 14:2158–2165
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Metwally, A., Shaaban, A.F., Azab, M.M. et al. Synthesis, characterization, morphology and adsorption performance towards cu+2 ions of nano-sized copolymers of anthranilic acid and o-aminophenol poly(anthranilic acid-co-o-aminophenol). J Polym Res 27, 90 (2020). https://doi.org/10.1007/s10965-019-1980-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1980-5