Abstract
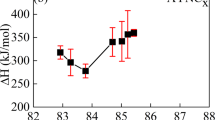
Glasses in the xAl2O3·(40 − x)Ag2O·60P2O5 system were prepared using melt quenching rout, where x varies between 0 and 20 mol%. Scanning electron microscopy indicates that the addition of Al2O3 has changed the morphology of the samples under investigation. The density of the glasses and the corresponding molar volume are both decreased linearly with increasing content of Al2O3. The dissolution rates (Dr) of the studied glasses were determined using weight loss method. Dr has showed a strong decreasing trend (from ~ 10−4 to ~ 10−9) upon increasing Al2O3 content. By increasing the concentration of Al2O3, the chemical durability of the investigated glasses is increased. Decreasing the weight loss was interpreted in terms of increasing concentration of P–O–Al bonds in the glasses investigated. The activation energy for ionic conduction showed an increasing behavior with increasing Al2O3 contents.







Similar content being viewed by others
References
J.A. Wilder Jr., J. Non-Cryst. Solids 38, 879 (1980)
R.K. Brow, J. Non-Cryst. Solids 263, 1 (2000)
L. Zhang, R.K. Brow, J. Am. Ceram. Soc. 94, 3123 (2011)
B. Sales, L. Boatner, J. Ramey, J. Non-Cryst. Solids 263, 155 (2000)
B.M.G. Melo, M.P.F. Graça, P.R. Prezas, M.A. Valente, A.F. Almeida, F.N.A. Freire, L. Bih, J. Non-Cryst. Solids 434, 28 (2016)
S.F. Khor, Z.A. Talib, W.M.M. Yunus, Ceram. Intern. 38, 935 (2012)
L. Baiaa, D. Muresan, M. Baiaa, J. Popp, S. Simon, Vib. Spectrosc 43, 313 (2007)
M. Lu, F. Wang, K. Chen, Y. Dai, Q. Liao, H. Zhu, Spect. Acta A 148, 1 (2015)
A. Maaroufi, O. Oabi, G. Pinto, M. Ouchetto, R. Benavente, J.M. Pereña, J. Non-Cryst. Solids 358, 2764 (2012)
J. Holubová, Z. Černošek, E. Černošková, L. Beneš, J. Therm. Anal. Calorim. 122, 47 (2015)
M.L. Marek Liška, A. Plško, M. Chromčíková, T. Gavenda, J. Macháček, J. Therm. Anal. Calorim. 121, 85 (2015)
J.H. Yang, H.S. Park, Y.Z. Cho, J. Nuclear Sci. Technol. 54, 1330 (2017)
P. Singh, S.S. Das, S.A. Agnihotry, J. Non-Cryst. Solids 351, 3730 (2005)
A. Faivre, D. Viviani, J. Phalippou, Solid State Ion. 176, 325 (2005)
J. Cui, H. Wen, S. Xie, W. Song, M. Sun, Yu Lin, Z. Hao, Mater. Res. Bull. 103, 70 (2018)
M. Saad, W. Stambouli, S.A. Mohamed, H. Elhouichet, J Alloys Compd. 705, 550 (2017)
J.L. Shaw, A.C. Wright, R.N. Sinclair, G.K. Marasinghe, D. Holland, M.R. Lees, C.R. Scales, J. Non-Cryst. Solids 345, 245 (2004)
H. Akamatsu, K. Fujita, S. Murai, K. Tanaka, Appl. Phys. Lett. 92, 251908 (2008)
L. Abbas, L. Bih, A. Nadiri, Y. El Amraoui, D. Mezzane, B. Elouadi, J. Mol. Struct. 876, 194 (2008)
C. Angell, Solid State Ion. 18, 72 (1986)
K. Hariharan, J. Maier, Solid State Ion. 86, 503 (1996)
K. Shaju, S. Chandra, Phys. Status Solid (B) 181, 301 (1994)
S.S. Das, B.P. Baranwal, C.P. Gupta, P. Singh, J. Power Sour. 114, 346 (2003)
R.I. Ainsworth, J.K. Christie, N.H. de Leeuw, Phys. Chem. Chem. Phys. 16, 21135 (2014)
S.P. Valappil, S.P. Valappil, D.M. Pickup, D.L. Carroll, C.K. Hope, J. Pratten, R.J. Newport, M.E. Smith, M. Wilson, J.C. Knowles, Antimicrob. Agen. Chemother. 51, 4453 (2007)
J. Swenson, A. Matic, C. Gejke, L. Börjesson, W.S. Howells, and M. J. Capitan Phys. Rev. B 60, 12023 (1999)
A.K. Varshneya, M. Tomozawa. J. Non-Cryst. Solids 170, 112 (1994)
E. Metwalli, R.K. Brow, J. Non-Cryst. Solids 289, 113 (2001)
S. Aqdim, A. Albizane, J. Greneche, J. Environ. Sci. Comput. Sci. Eng. Technol. 4, 509 (2015)
G. El-Damrawi, A.K. Hassan, A. Shahboub, Mag. Reson. Solids 20, 18202 (2018)
J. Egan, R. Wenslow, K. Mueller, J. Non-Cryst. Solids 261, 115 (2000)
L. Zhang, H. Eckert, J. Phys. Chem. B 110, 8946 (2006)
M. Karabulut, E. Metwalli, R.K. Brow, J. Non-Cryst. Solids 283, 211 (2001)
G. El-Damrawi, A.K. Hassan, H. Doweidar, A. Shahboub, New J. Glas. Ceram. 7, 77 (2017)
K.M. Shaju, S. Chandra, Phys. Stat. Sol. (B) 181, 301 (1994)
M.D. Ingram, M.A. Mackenzie, W. Müller, M. Torge, Solid State Ion. 28, 677 (1988)
D.E. Day, Z. Wu, C.S. Ray, P. Hrma, J. Non-Cryst. Solids 241, 1 (1998)
H. Gao, T. Tan, D. Wang, J. Cont. Rel. 96, 29 (2004)
S.T. Reis, M. Karabulut, D.E. Day, J. Non-Cryst. Solids 292, 150 (2001)
G. Perera, R.H. Doremus, W. Lanford, J. Am. Ceram. Soc. 74, 1269 (1991)
Acknowledgments
The authors wish to express their grateful thanks to Asst. Prof. Ph.D. Eng. Dumitru CHIRLESAN, Rector of UPIT, Romania, for his kind help and encouragement which he extended to us throughout the period of this study. Further, the authors are highly indebted to Dr. Catalin Ducu head of (CRC&D-Auto), UPIT, Romania, and his colleagues for giving us the opportunity to do research and providing invaluable guidance throughout this research.
Author information
Authors and Affiliations
Contributions
GED was involved in conceptualization, supervision, writing—review and editing. AKH contributed to writing—review and editing. AS was involved in methodology, validation, investigation, writing—original draft, visualization.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Damrawi, G., Hassan, A.K. & Shahboub, A. Chemical durability and structure of Al2O3–Ag2O–P2O5 glasses. Appl. Phys. A 126, 271 (2020). https://doi.org/10.1007/s00339-020-3451-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-3451-6