Abstract
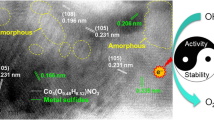
Developing highly efficient, low-cost, and stable electrocatalyst for the oxygen evolution reaction (OER) is essential for hydrogen production by electrolyzing water. In this paper, ZnO/amorphous-FeOOH core-shell nanorod array is fabricated on Ni foil via a simple two-step chemical bath method and used as OER catalyst. Amorphous FeOOH possesses more O defects than crystalline FeOOH. Besides, the X-ray photoelectron spectroscopy (XPS) results reveal that the strong electron interactions on nano-interface between ZnO and FeOOH facilitate charge transfer from FeOOH to ZnO. Due to these advantages, the ZnO/amorphous-FeOOH hybrid nanorods exhibit excellent electrocatalytic performance for OER, with an overpotential of 301 mV at 10 mA cm−2, Tafel slope of 50 mV dec−1, and outstanding stability.








Similar content being viewed by others
References
Wu LK, Hu JM (2014) A silica co-electrodeposition route to nanoporous Co3O4 film electrode for oxygen evolution reaction. Electrochim Acta 116:158–163
Zhu SS, Huang LA, He ZS, Wang K, Guo JF, Pei SE, Shao HB, Wang JM (2018) Investigation of oxygen vacancies in Fe2O3/CoOx composite films for boosting electrocatalytic oxygen evolution performance stably. J Electroanal Chem 827:42–50
Cherevko S, Geiger S, Kasian O, Kulyk N, Grote J-P, Savan A, Shrestha BR, Merzlikin S, Breitbach B, Ludwig A, Mayrhofer KJJ (2016) Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: a comparative study on activity and stability. Catal Today 262:170–180
Zhang Y, Jia G, Wang H, Ouyang B, Rawat RS, Fan HJ (2017) Ultrathin CNTs@FeOOH nanoflake core/shell networks as efficient electrocatalysts for the oxygen evolution reaction. Mater Chem Front 1:709–715
He Q, Xie H, Rehman ZU, Wang C, Wan P, Jiang H, Chu W, Song L (2018) Highly defective Fe-based oxyhydroxides from electrochemical reconstruction for efficient oxygen evolution catalysis. ACS Energy Lett 3:861–868
Chi J, Yu H, Jiang G, Jia J, Qin B, Yi B, Shao Z (2018) Construction of orderly hierarchical FeOOH/NiFe layered double hydroxides supported on cobaltous carbonate hydroxide nanowire arrays for a highly efficient oxygen evolution reaction. J Mater Chem A 6:3397–3401
Zhang B, Wang L, Zhang Y, Ding Y, Bi Y (2018) Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angew Chem Int Ed 57(8):2248–2252
Wang K, Chen MM, He ZS, Huang LA, Zhu SS, Pei SE, Guo JF, Shao HB, Wang JM (2018) Hierarchical Fe3O4@C nanospheres derived from Fe2O3/MIL-100(Fe) with superior high-rate lithium storage performance. J Alloys Compd 755:154–162
Li F, Du J, Li X, Shen J, Wang Y, Zhu Y, Sun L (2018) Integration of FeOOH and zeolitic imidazolate framework-derived nanoporous carbon as an efficient electrocatalyst for water oxidation. Adv Energy Mater 8:1702598–1702602
Chen G-F, Luo Y, Ding L-X, Wang H (2017) Low-voltage electrolytic hydrogen production derived from efficient water and ethanol oxidation on fluorine-modified FeOOH anode. ACS Catal 8:526–530
Meng H, Ren Z, Du S, Wu J, Yang X, Xue Y, Fu H (2018) Engineering a stereo-film of FeNi3 nanosheet-covered FeOOH arrays for efficient oxygen evolution. Nanoscale 10(23):10971–10978
Xie M, Xiong X, Yang L, Shi X, Asiri AM, Sun X (2018) An Fe(TCNQ)2 nanowire array on Fe foil: an efficient non-noble-metal catalyst for the oxygen evolution reaction in alkaline media. Chem Commun 54(18):2300–2303
Vassalini I, Borgese L, Mariz M, Polizzi S, Aquilanti G, Ghigna P, Sartorel A, Amendola V, Alessandri I (2017) Enhanced electrocatalytic oxygen evolution in au-Fe nanoalloys. Angew Chem Int Ed 56(23):6589–6593
Zhang X, Zhang B, Liu S, Kang H, Kong W, Zhang S, Shen Y, Yang B (2018) RGO modified Ni doped FeOOH for enhanced electrochemical and photoelectrochemical water oxidation. Appl Surf Sci 436:974–980
Han X, Yu C, Yang J, Song X, Zhao C, Li S, Zhang Y, Huang H, Liu Z, Huang H, Tan X, Qiu J (2019) Electrochemically driven coordination tuning of FeOOH integrated on carbon fiber paper for enhanced oxygen evolution. Small 15:1901015–1901023
Li M, Tao L, Xiao X, Lv X, Jiang X, Wang M, Peng Z, Shen Y (2018) Core-shell structured NiCo2O4@FeOOH nanowire arrays as bifunctional electrocatalysts for efficient overall water splitting. ChemCatChem 10:4119–4125
Niu S, Jiang WJ, Wei Z, Tang T, Ma J, Hu JS, Wan LJ (2019) Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation. J Am Chem Soc 141(17):7005–7013
Feng JX, Xu H, Dong YT, Ye SH, Tong YX, Li GR (2016) FeOOH/Co/FeOOH hybrid nanotube arrays as high-performance electrocatalysts for the oxygen evolution reaction. Angew Chem Int Ed 55(11):3694–3698
Indra A, Menezes PW, Sahraie NR, Bergmann A, Das C, Tallarida M, Schmeisser D, Strasser P, Driess M (2014) Unification of catalytic water oxidation and oxygen reduction reactions: amorphous beat crystalline cobalt iron oxides. J Am Chem Soc 136:17530–17536
Chen G, Zhou W, Guan D, Sunarso J, Zhu Y, Hu X, Zhang W, Shao Z (2017) Two orders of magnitude enhancement in oxygen evolution reactivity on amorphous Ba0.5Sr0.5Co0.8Fe0.2O3−δ nanofilms with tunable oxidation state. Sci Adv 3:1603206–1603213
Cai Z, Li L, Zhang Y, Yang Z, Yang J, Guo Y, Guo L (2019) Amorphous nanocages of Cu-Ni-Fe Hydr(oxy)oxide prepared by photocorrosion for highly efficient oxygen evolution. Angew Chem Int Ed 58(13):4189–4194
Huang J, Chen J, Yao T, He J, Jiang S, Sun Z, Liu Q, Cheng W, Hu F, Jiang Y, Pan Z, Wei S (2015) CoOOH nanosheets with high mass activity for water oxidation. Angew Chem Int Ed 54(30):8722–8727
Guo C, Sun X, Kuang X, Gao L, Zhao M, Qu L, Zhang Y, Wu D, Ren X, Wei Q (2019) Amorphous Co-doped MoOx nanospheres with a core–shell structure toward an effective oxygen evolution reaction. J Mater Chem A 7:1005–1012
Duan Y, Yu ZY, Hu SJ, Zheng XS, Zhang CT, Ding HH, Hu BC, Fu QQ, Yu ZL, Zheng X, Zhu JF, Gao MR, Yu SH (2019) Scaled-up synthesis of amorphous NiFeMo oxides and their rapid surface reconstruction for superior oxygen evolution catalysis. Angew Chem Int Ed 58(44):15772–15777
Zhang LY, Li H, Yang BW, Ying Z, Zhang ZT, Wang Y (2019) Photo-deposition of ZnO/Co3O4 core-shell nanorods with p-n junction for efficient oxygen evolution reaction. J Solid State Electr 23:3287–3297
Yang S, Li Y, Xu T, Li Y, Fu H, Cheng K, Ye K, Yang L, Cao D, Wang G (2016) FeOOH electrodeposited on Ag decorated ZnO nanorods for electrochemical energy storage RSC Adv 6:39166–39171
Stevens MB, Enman LJ, Batchellor AS, Cosby MR, Vise AE, Trang CDM, Boettcher SW (2016) Measurement techniques for the study of thin film heterogeneous water oxidation electrocatalysts. Chem Mater 29:120–140
Xia D, Quan J, Wu G, Liu X, Zhang Z, Ji H, Chen D, Zhang L, Wang Y, Yi S, Zhou Y, Gao Y, Jin RH (2019) Linear-polyethyleneimine-templated synthesis of N-doped carbon nanonet flakes for high-performance supercapacitor electrodes. Nanomaterials 9:1225–1237
Wang H-L, Cui J-Y, Jiang W-F (2011) Synthesis, characterization and flocculation activity of novel Fe(OH)3–polyacrylamide hybrid polymer. Mater Chem Phys 130:993–999
Lee J, Lee H, Lim B (2018) Chemical transformation of iron alkoxide nanosheets to FeOOH nanoparticles for highly active and stable oxygen evolution electrocatalysts. J Ind Eng Chem 58:100–104
Sankar Ganesh R, Durgadevi E, Navaneethan M, Patil VL, Ponnusamy S, Muthamizhchelvan C, Kawasaki S, Patil PS, Hayakawa Y (2017) Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres. J Alloys Compd 721:182–190
Ye SH, Shi ZX, Feng JX, Tong YX, Li GR (2018) Activating CoOOH porous nanosheet arrays by partial iron substitution for efficient oxygen evolution reaction. Angew Chem Int Ed 57(10):2672–2676
Li H, Zhou Q, Liu F, Zhang W, Tan Z, Zhou H, Huang Z, Jiao S, Kuang Y (2019) Biomimetic design of ultrathin edge-riched FeOOH@carbon nanotubes as high-efficiency electrocatalysts for water splitting. Appl Catal B Environ 255:117755–117763
Luo W, Jiang C, Li Y, Shevlin SA, Han X, Qiu K, Cheng Y, Guo Z, Huang W, Tang J (2017) Highly crystallized α-FeOOH for a stable and efficient oxygen evolution reaction. J Mater Chem A 5:2021–2028
Gong Y, Pan H, Xu Z, Yang Z, Lin Y, Zhang M (2018) ACo2O4 (a=Ni, Zn, Mn) nanostructure arrays grown on nickel foam as efficient electrocatalysts for oxygen evolution reaction. Int J Hydrog Energy 43:14360–14368
He D, Xiong Y, Yang J, Chen X, Deng Z, Pan M, Li Y, Mu S (2017) Nanocarbon-intercalated and Fe-N-codoped graphene as a highly active noble-metal-free bifunctional electrocatalyst for oxygen reduction and evolution. J Mater Chem A 5:1930–1934
Fan X, Kong F, Kong A, Chen A, Zhou Z, Shan Y (2017) Covalent porphyrin framework-derived Fe2P@Fe4N-coupled nanoparticles embedded in N-doped carbons as efficient trifunctional electrocatalysts. ACS Appl Mater Interfaces 9(38):32840–32850
Xie M, Xiong X, Yang L, Shi X, Asiri AM, Sun X (2018) Fe(TCNQ)2 nanowires array on Fe foil: an efficient non-noblemetal catalyst for the oxygen evolution reaction in alkaline media. Chem Commun 54(18):2300–2303
Xiong D, Wang X, Li W, Liu L (2016) Facile synthesis of iron phosphide nanorods for efficient and durable electrochemical oxygen evolution. Chem Commun 52(56):8711–8714
Suho J, Charles C, Ivonne MF, Jonas CP, Thomas FJ (2016) Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J Mater Chem A 4:3068–3076
Xue Z, Li X, Liu Q, Cai M, Liu K, Liu M, Ke Z, Liu X, Li G (2019) Interfacial electronic structure modulation of NiTe nanoarrays with NiS nanodots facilitates electrocatalytic oxygen evolution. Adv Mater 31:1900430–1900436
Funding
This study was supported by the Science and Technology Department of Henan Province (no. 162300410028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2409 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Li, H., Yang, B. et al. Promote the electrocatalysis activity of amorphous FeOOH to oxygen evolution reaction by coupling with ZnO nanorod array. J Solid State Electrochem 24, 905–914 (2020). https://doi.org/10.1007/s10008-020-04540-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04540-2