Abstract
Biofouling of PVAc and PVOH surfaces by fungal conidia can result in surface discolouration and subsequent biodeterioration. In order to understand the interactions of fungal conidia on polymer surfaces, the surface properties of PVAc and PVOH and the hydrophobicity, size and shape of three type of fungal conidia was determined (Aspergillus niger 1957, Aspergillus niger 1988 and Aureobasidium pullulans). Fungal conidia were used in a range of binding assays (attachment, adhesion and retention). The PVAc and PVOH demonstrated different surface topographies and the PVAc demonstrated a higher maximum height (300.6 nm) when compared to the PVOH (434.2 nm). The PVAc surfaces was less wettable (75°) than the PVOH surface (62°). The FTIR demonstrated differences in the chemistries of the two surfaces, whereby the PVOH confirmed the presence of polar moieties. Hydrophobicity assays demonstrated that both A. niger species’ were more non-wettable than the A. pullulans. Following the attachment assays, the more hydrophobic Aspergillus spp. conidia attached in greater numbers to the more wettable surface and the A. pullulans was retained in greater numbers to the less wettable PVAc surface. The adhesion and retention assays demonstrated that the more polar surface retained all the types of conidia, regardless of their surface hydrophobicities. This study demonstrated that conidial binding to the surfaces were influenced by the chemistry and physicochemistry of the surfaces and spores. However, the inclusion of a washing stage influenced the adhesion of conidia to surfaces. In environments that were indicative of a attachment or retention assay a PVAc surface would reduce the number of A. niger spp. spores whilst a PVOH surface would reduce the number of A. pullulans spores. However, in an environment similar to a adhesion assay, a PVAc surface would be most beneficial to reduce spore retention. Thus, the use of the correct methodology that reflects the environment in which the surface is to be used is important in order to accurately inform hygienic surface development.
Similar content being viewed by others
Introduction
The biofouling of surfaces by fungal spores may result in undesired effects such as their colouring and deterioration [1, 2] or may be beneficial to enhance biotechnological processes, such as the biodeterioration of polymer wastes [3], or malic acid production from biodiesel using Aspergillus niger [4]. However, degradation caused by fungi, is a major issue with the application of polymeric materials [5]. Fungi can degrade polymers over time via a number of mechanisms, which include enzymatic activity and physical disruption, which results in a reduction of plasticizers within the polymer composite causing damaged, weakened and unsightly materials [6].
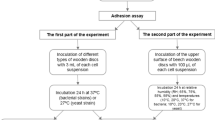
The binding of fungal conidia to surfaces is influenced by both the properties of the surface and of the fungal spores [2]. The binding of fungal spores to a surface is influenced by many factors such as surface topography, chemistry, physicochemistry and electrostatic interactions [2, 7,8,9,10]. However, the interactions at the cell:substratum interface may also be influenced by the experimental methodology. In this work, the microbial attachment was defined as spore attachment to the surfaces that was initially reversible [11]. However, since cells normally become irreversibly attached after a short contact period, a rinse step was added after cell attachment to determine the number of cells that adhered to the surface; this was defined as the adhesion assay. The retention assay incorporated a one hour submersion time of the substrata in the spore suspension and a wash step, so that the spores were retained on the surface. Understanding of the spore:surface interactions that affect increased or decreased spore binding may help in the development of surfaces to understand and control the conidial binding mechanisms [8].
Poly(vinyl acetate) (PVAc) is an aliphatic synthetic polymer which is often used as an thermoplastic adhesive [12]. It is a non-toxic, commercially important polymer which is prepared through emulsion polymerisation [13]. Poly(vinyl alcohol) (PVOH) is the most widely produced water soluble synthetic polymer, which is made from poly(vinyl acetate) via hydrolysis [14]. Due to its many desirable properties including high mechanical strength, transparency, excellent gas barrier characteristics and biodegradability [15], it is widely used in a multitude of industries including the industrial, commercial, medical and food sectors [16]. Due to the versatile nature of both polymers they are used extensively in many household fabrics and surfaces, but their degradation cycles are limited with plastic accounting for at least 7.4% of municipal solid waste in Western Europe [17].
The genus Aspergillus is a filamentous ascomycete fungus and is among the most abundant on Earth, due to their tolerance towards a range of environments e.g. low humidities and a wide range of temperatures (6–55 °C) [18]. The utilisation of a wide range of organic conditioning films as nutrient sources, coupled with the vast number of conidia released by Aspergillus colonies has contributed to the success of this genus [18]. Aspergillus niger has a long history of safe use for enzyme production and number of secondary metabolites [19]. Environmentally, the risk of exposure to Aspergillus spp. spores is dependent on climatic conditions such as humidity, temperature and wind [20]. Due to the production of spores, A. niger can remain in the atmosphere for prolonged periods of time until an opportunity to germinate arises, such as stimulation by high surrounding nutrient levels and a pH > 5 [21]. A. niger can cause opportunistic infections of humans [22]. However, the host defence systems of a healthy human can deal with this threat. However, in individuals that are immunosuppressed, invasive aspergillosis almost always occurs [23]. Due to their presence in the environment, fungal conidia are inhaled on a continual basis. A. fumigatus is the most invasive aspergillosis, but there is now an increasing incidence of infections caused by antifungal resistant non-fumigatus spp. [24,25,26,27]. In immunosuppressed individuals the inhalation of Aspergillus conidia. can produce invasive infections, which are frequently associated with high morbidity and mortality [23].
A. pullulans is a ubiquitous saprophytic fungus that can be a beneficially microbe since it can produce a wide range of enzymes and thus it has a wide range of biotechnologically important applications [28]. Different strains of A. pullulans have been shown to act as a host–pathogen–antagonist in the control of other fungal species, and such properties have potential for use in controlling the spoilage of fruits by other moulds [29]. A. pullulans produces its chlamydospores from swollen cells or from septate swollen cells, and it has a thick cell wall and they may be partially or fully covered with melanin [30]. In addition, the spores are the major source of a polysaccharide pullulan [31]. Such morphology means that these cells can withstand air drying without collapse, and they have also been shown to be resistant to ultraviolet irradiation [32]. The conidia of this deteriogenic fungus can bind to substances resulting in biodegradation over time [33]and this is especially common in domestic settings, such as on bathroom surfaces and synthetic polymers [6, 34].
In order to understand the interactions between PVAc and PVOH surfaces and fungal spores, the hydrophobicity of three variants of fungal conidia (A. niger strain 1957, A. niger strain 1988 and A. pullulans strain) was determined. Furthermore, surface properties (topography, friction, surface free energies and physicochemical) of two polymeric surfaces, PVAc and PVOH, were evaluated. Attachment, adhesion and retention assays were used to identify the nature of the interactions between fungal conidia and polymer surfaces, with a view to optimising surface properties to reduce colonisation.
Methods and Materials
Compression Moulding
A hydraulic press (Press type 202B-50 ton Bradley and Turton Ltd., UK) fitted with induction heated platens was used to compression mould PVAc (Sigma, UK) at 150 °C and commercially hydrolysed polyvinyl acetate (PVOH) (Sigma, UK) at 220 °C (after pre-softening for 30 min at 250 °C). A steel frame mould (dimensions: 16.3 cm × 19.5 cm (outside), 8.8 cm × 15.4 cm (inside) and 0.1 cm thick) was used in conjunction with fluorinated ethylene propylene (FEP) release sheets and stainless steel (30.2 cm × 23 cm × 0.1 cm) mould plates. The mould was pre-heated (5 min), before removal from the press and charging with the polymer granules and returning to the press, followed by compressing at full pressure for 10 min. The mould was then immediately transferred into a cold press (Francis Shaw and Company, UK) for 5 min. The mould was then removed from the cold press and the moulding released. The pressure applied to the polymer melt in the mould before closure was estimated to be between 2.9 and 3.7 MPa, assuming clamping forces before closure of 40 kN and 50 kN respectively, and a mould projected area of 0.136 m2. It must be noted that once a frame mould is closed, no further pressure can be applied to the polymer melt.
Scanning Electron Microscopy (SEM)
Surfaces were gold sputter coated using an SEM sputter coater (Polaron E5100 UK) set to the following parameters: power 18–20 mA, 3 min, 2500 V, vacuum 0.09 mbar, argon gas and then imaged using a JEOL JSM 5600LV scanning electron microscope (n = 9).
Determination of Surface Roughness, Topography and Friction
Substrata images and roughness measurements were obtained using an Explorer Atomic Force Microscope (AFM) (Veeco, UK). Analysis was carried out using a cantilever with a spring constant of 0.12 N m−1 in contact mode. Roughness values Ra, root mean squared (RMS), maximum height, average height and surface area were determined and the frictional properties of the surface were carried out using the phase imaging mode of the AFM (n = 3).
Perpendicular Force Measurements of Surface Heterogeneity
The AFM cantilever was used to determine the perpendicular force between the cantilever tip and the surface. The spring constant of the cantilever was determined before each measurement. The cantilever tip was brought into contact with the surface, and the strength of attachment was obtained from force–distance curves [2]. Using Hooke’s Law, he zero of the force, spring constant of the cantilever, and the cantilever deflection (d) were converted into a force (F) [35, 36] whereby;
where the distance (d) was determined as a function of (z–d), where z was the displacement of the piezoelectric scanner in a vertical direction, and k was the cantilever spring constant. The spring constant was multiplied by the displacement, and the zero of the force was subtracted from the setpoint, and converted to nN from nA (n = 20).
Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
ATR-FTIR was used to determine the molecular structures and chemical bonds of the PVC and PVOH surfaces (Nicolet 380 FTIR with a Smart iTR attachment (with diamond internal reflection element), Thermo Scientific, UK). Background spectra was captured prior to each measurement and spectra were acquired at room temperature using Omnic 5.2 software with each run made up of 16 scans and a resolution of 4 cm−1. Analysis of each sample was performed in triplicate and the average spectra were reported (n = 3).
Surface Free Energies
The contact angle and surface free energy components were determined using Dynamic contact angle analysis (DCA 322-1, Cahn Instruments, USA) [37, 38]. Contact angle measurements of clean, dry substrata were taken in HiPerSolv HPLC grade H2O, diiodomethane and formamide (n = 6).
Fungal Spore Assays
The selection of the strains was determined by their differences in wettability and their differences in shape, as it is known that such factors will influence spore attachment to the surfaces [2].
Spore Suspensions
Fungi were grown using Sabouraud (SAB) broth or agar (Lab M, UK). Inoculated plates were incubated for 3–21 days at 29 °C. Aliquots of 5 mL SAB were added to the fungal culture and spores were removed using a glass Pasteur pipette with gentle agitation over the surface of the culture and repeated as needed. Spore suspensions were stirred for 30 min and filtered through glass wool (VWR, UK) to obtain a homogeneous spore suspension. Spores were harvested by centrifugation for 10 min at 1721 g, washed in distilled water and adjusted to an OD610 of 1.0 (± 0.1). Spore counts were determined using a haemocytometer.
Imaging of Fungal Spores
Ten microliters of the washed fungal conidia were deposited onto a glass microscopy slide. The spores were imaged using a Leitz microscope (UK). To prepare the conidia for SEM, the 10 µL of spore suspension was placed onto a 10 mm × 10 mm piece of polished silicon wafer (Montco Technologies, USA) and dried at room temperature for 1 h in a class 2 laminar flow hood. The samples with dried spores were fixed in 4% v/v glutaraldehyde for 24 h at 4 °C and were rinsed with sterile distilled water. The fixed spores were passed through an absolute ethanol gradient from 10, to 30%, 50%, 70%, 90% and 100% v/v ethanol, to remove water. The samples were stored in a dessicator until visualisation. Before imaging using the SEM, the samples were coated using a Polaron gold sputter coated for 30 s.
Phase Exclusion Assay
Toluene (BDH, UK) and spore suspensions (OD610 0.6) were mixed in 1:1 ratios, vortexed then rested for 30 min. The absorbance of the aqueous phase was measured and the results expressed as the proportion of the cells which were excluded from the aqueous phase using the equation 100 × (Ai–Af)/Ai, where Ai is the initial optical density of the aqueous phase and Af is the final optical density of the aqueous phase [39, 40].
Bonding of Spore Assays
Adhesion Assay
Substrata sized 10 mm × 10 mm were attached using adhesive gum (Impega, Malaysia) to a vertical stainless steel tray. Using a Badger Airbrush (Shesto, UK), propelled by a Letraset 600 mL liquid gas canister (Esselte Letraset Ltd, UK) the spore suspension (OD610 1.0) was placed into the spray reservoir and passed ten times from left to right over the surfaces at a distance of 10 cm, speed of 50 mm s−1 (flow rate of 0.2 mL s−1) per pass (n = 6).
Attachment Assay
The attachment assay was repeated as above, except that immediately following spraying, substrata were held vertically and rinsed once, to gently remove loosely attached spores. 5 mL distilled water was dispensed at a 45° angle, with a 3 mm nozzle. The substrata with the retained spores were laid horizontally and aseptically air-dried for 1 h (n = 6).
Retention Assay
Substrata were placed in sterile glass Petri dishes and 25 mL of spore suspension was added and incubated without agitation for 1 h. Substrata were then washed gently with 10 mL distilled water and were aseptically air-dried for 1 h (n = 6) [41].
Imaging of Spores
The spores on the substrata were stained using either 3% crystal violet, or 0.03% acridine orange in 2% glacial acetic acid (Sigma, UK) for 2 min, rinsed and air-dried. Spores were visualised using either light or epifluorescence microscopy (Nikon Eclipse E600, Nikon, UK). The number of spores per cm2 or % coverage was determined (n = 60).
Statistical Analysis
The results were statistically analysed using ANOVA and T-tests. Data was considered significant when p < 0.05. data was analysed using Excel. Error bars represent the standard error of the mean.
Results
Surface Properties
This work was carried out to determine the effect of the spore hydrophobicity and chemistry on their attachment, adhesion and retention onto two poly vinyl based surfaces.
Surface Topography and Frictional Force
Scanning electron microscopy was used to image the overall surface roughness of each substratum at low magnification (Fig. 1a, b). It was demonstrated that both surfaces contained striations across the surface between 1 and 10 µm, which were possibly due to the manufacturing process. The PVAc demonstrated unidirectional lines across the surface (Fig. 1a) whilst the PVOH demonstrated surface striations that were more varied in direction (Fig. 1b).
At higher magnification, it was observed that the PVAc (Fig. 1c) had a more irregular surface topography (320.9 nm) than the PVOH (301.3 nm) (Fig. 1e). From the phase analysis images of the PVAc and PVOH surfaces (Fig. 1d, f respectively), it was demonstrated that the PVAc had greater differences in the frictional properties of the material as evidenced by the greater differences in the light and dark areas. The darker areas correspond to areas of the polymer surface whereby the AFM cantilever has been ‘stuck’ and required greater force to move across the surface, which is indicative of an area of greater resistance.
The heterogeneity of the surface chemistry was determined using the measurement of the force of interaction of an AFM cantilever onto the surface of the polymer (Fig. 2). The results demonstrated that the PVAc surface had a smaller first and third quartile of measurements. However, the overall range of attachment measurements was larger for the PVAc surface (1.8–38.8 nN) than for the PVOH surface (4.0–32.7 nN), demonstrating that the PVAc surface had greater chemical heterogeneity across the surface. This meant that the cantilever required a greater range of forces to be removed from the surface.
Although there was a difference visually in the surface topographies of the two materials, the qualitative results were not significantly different for the Ra (average mean centre line) (PVAc 45.7 nm; PVOH 39.6 nm), RMS (the standard deviation of the heights; PVAc 61.7 nm; PVOH 51.8 nm), average height (PVAc 300.6 nm; PVOH 434.3 nm) or surfaces area (PVAc 422.3 µm; PVOH 423.5 µm) values (Fig. 3) (Data not shown for surface area values). However, there was a significant difference in the maximum height recorded for the two surfaces, with the PVAc having a higher range (548.0 nm) than the PVOH surface (251.9 nm) (Fig. 3).
Surface Wettabilities
Surface analysis demonstrated that the PVAc surfaces were less wettable (77.9°) than the PVOH surface (61.9°) (Fig. 4). The surface free energies followed the opposite trend, whereby the surface free energy of the PVAc surfaces (35.4 mJ/m2) was lower than that of the PVOH (41.0 mJ/m2). Although there was no difference in the dispersive components of the surfaces (PVAc 28.5 mJ/m2 and 29 mJ/m2 respectively), the polar surface component was lower for the PVAc surface (7.0 mJ/m2) than the PVOH surface (11.9 mJ/m2). This demonstrated that the PVAc surface was less wettable, and demonstrated less polar moieties on the surface.
Surface Chemistry
ATR-FTIR spectra featured all the absorption peaks associated with these polymers (Fig. 5). The spectrum of PVAc was dominated by the ester carbonyl stretching vibration at 1731 cm−1 and the C(=O)–O stretching band at 1230 cm−1. The C–H stretching and deformation bands were centred at 2924 cm−1, and at 1433 cm−1/1375 cm−1, respectively. The bands appearing below 1230 cm−1 may be skeletal stretching modes.
The ATR-FTIR spectrum of the PVOH was dominated by the broad hydrogen bonded OH stretching band, centred at 3272 cm−1 and the C–H stretching bands centred at 2913 cm−1. The fingerprint region of PVOH features the C–O stretching band at 1092 cm−1, and the accompanying O–H bending coupled C–H vibrations at 1418 cm−1 and 1329 cm−1. The other interesting band was the carboxylate carbonyl stretch at 1570 cm−1 which may be assigned to sodium acetate, possibly produced as a result of reaction between the sodium methoxide alcoholysis catalyst and the acetic acid used to neutralise the reaction mixture (Saunders, 1985). The weaker band at ca. 1730 cm−1 (not peak picked but present) may be assigned to residual ester (the level of PVAc hydrolysis is never exactly 100%). The band at 1660 cm−1 may be assignable to C=C stretching from unsaturation arising from degradation during synthesis.
Fungal Spore Assays
The fungal spores were analysed in terms of their shape and surface wettabilities.
Spore Morphology
The shapes of the conidia were analysed using light microscopy and SEM, and it was determined that the A. niger 1957 conidia were spherical in shape, and around 5 µm in size (Fig. 6a). The A. niger 1988 conidia were 5 µm to 8 µm in size, but had regular, spikey protrusions form the surface, around 0.5 µm in length (Fig. 6b). The A. pullulans conidia was more varied in size, but generally ranged from 5 to 12 µm in length and around 3 µm to 4 µm in width (Fig. 6c).
Spore Wettability
Polar/non polar solvent assays were used to determine the wettability of the fungal spores. It was demonstrated that both the A. niger species were more non-wettable (A. niger 1957, 11.7%; A. niger 1988, 9.2%) than the A. pullulans species (73.6%) (Fig. 7).
Visualisation Following Spore Assays
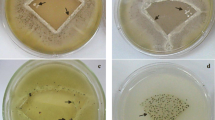
Attachment (Fig. 8), adhesion (Fig. 9) and retention assays (Fig. 10) were carried out to determine the number of conidia retained. The adhesion and retention assays both included washing steps of the surfaces to remove any loosely bound conidia.
Following imaging of the attachment assays, the work demonstrated that visually, it did not seem as if conidia attachment was influenced by the striations seen on the surfaces using SEM. The A. niger 1957 spores looked to be more clumped together (Fig. 8a, d) than the A. niger 1988 spores (Fig. 8b, e), regardless of the underlying surface properties. The A. pullulans conidia on the PVAc surfaces were clumped together, (Fig. 8c), whilst on the PVOH surface, the spores were not as grouped together (Fig. 8f).
The adhesion assays demonstrated that on the PVAc surface the A. niger 1957 and A. niger 1988 spores that could be seen were paired together (Fig. 9a, b). On the PVOH surfaces, the A. niger 1957 and A. niger 1988 spores looked more homogeneous in spread (Fig. 9d, e). The A. pullulans looked to be well spread out on the PVAc surface, but was in small clumps on the PVOH surface (Fig. 9c, f).
Following the retention assay, the A. niger 1957 and A. niger 1988 spores looked to be homogeneously distributed on the PVAc (Fig. 10a, b) and the PVOH surface (Fig. 10d, e). The A. pullulans spores did not look to be grouped together on either the PVAc (Fig. 10c) or the PVOH (Fig. 10f) surfaces following the retention assays.
Attachment, Adhesion and Retention Assays
Following the initial attachment assay, it was demonstrated that both A. niger species were retained in significantly lower numbers on the PVAc surface (strain 1957, 1.02 × 105 cm−2 and strain 1988, 3.56 × 104 cm−2) than on the PVOH surfaces (strain 1957, 2.17 × 105 cm−2 and stain 1988, 1.36 × 105 cm−2) (Fig. 11a). However, the opposite trend was demonstrated for the A. pullulans whereby 3.15 × 105 cm−2 conidia were attached to the PVAc surface and 2.83 × 104 cm−2 conidia were attached to the PVOH surface. This demonstrated that for the attachment assays, the more hydrophobic spores were attached in greater numbers to the more wettable surface.
Following the adhesion assays, a different trend was observed. All the conidia types were adhered in greatest numbers to the PVOH surface (Fig. 11b). On the PVAc surface 3.75 × 102 cm−2, 1.50 × 103 cm−2 and 2.81 × 102 cm−2 condida had ahered for A. niger 1957, A. niger 1988 and A. pullulans respectively. In comparison, on the PVOH surface, 4.22 × 104 cm−2 3.00 × 103 cm−2 and 9.36 × 102 cm−2 conidia had adhered for A. niger 1957, A. niger 1988 and A. pullulans respectively. This demonstrated that following the adhesion assays the more polar surface retained all the types of conidia, regardless of their surface hydrophobicities.
Following the retention assays, the A. niger 1957 and A. niger 1988 were retained in the greatest numbers on the PVOH surfaces (3.50 × 105 cm−2 on PVOH compared to 2.56 × 104 cm−2 on PVAc for A. niger 1957; 1.80 × 105 cm−2 on PVOH compared to 1.27 × 105 cm−2 on PVAc for A. niger) whilst the A. pullulans was retained in the greatest numbers on the PVAc surface (1.35 × 104 cm−2) when compared to the PVOH surface (5.24 × 103 cm−2) (Fig. 11c). Following the retention assays, the most hydrophobic spores were retained in the greatest numbers to the more wettable surfaces.
Discussion
PVAc and PVOH are commercially important polymers which are susceptible to fungal biofouling which leads to discolouration and subsequent biodegradation due to loss of structural integrity [42]. Once conidia have attached to a surface, they become adhered and then retained [43]. These steps often result in the germination of mycelia which may lead to the formation of biofilms on the polymer surface [44, 45]. Throughout this study, the surface properties (topography, chemistry and wettability) of two polymers, PVAc and PVOH, were evaluated to determine their effects on the binding wettable and non-wettable, differently shaped fungal conidia.
The SEM images demonstrated that both surfaces contained striations across the surface, which were possibly due to the manufacturing process. However, when the distribution of the conidia across the surface was visualised following the spore binding assays, the surface features did not seem to influence the pattern of spore retention in any of the assays used. Thus, at this level, the topographical features did not influence the pattern of spore retention. It has been demonstrated that surface features may influence the pattern of retention [46], whilst other work has demonstrated that the surface and cell physicochemistry has a greater effect [47]. However, others have shown that the size of the surface features influences surface retention [48, 49]. Yet in agreement with results from Whitehead et al. [50], the striations of the surfaces did not affect the pattern of conidia retention.
AFM was used to determine the topographical features at a higher magnification. The AFM showed a heterogeneous surface topography where the PVAc had a more irregular surface topography than the PVOH. The main parameter often reported in the literature for attachment and adhesion of microorganisms is surface roughness, and this may be evaluated according to R or S values [46, 51]. It has been suggested that a rougher surface may enhance the attachment and adhesion of fungi, due to the greater surface area and therefore more available surface sites for thermodynamic reactions to occur [52, 53]. However, if topographical features are considerably larger than the microorganisms in question then retention may not be significant [54]. With the exception of the surface area, the surface roughness values evaluated using the AFM were below that of one micron, and hence well below the size of the fungal conidia. However, there was no significant differences in the roughness values between the two surfaces, with the exception of the maximum height, whereby the PVAc surface demonstrated a greater height value. With the exception of the A. pullulans attachment and retention assay, and the A. niger 1988 retention assay, the fungal conidia were bound in greater numbers on the PVOH surface, rather than the PVAc surface suggesting that the surface chemistry and physicochemistry had a greater effect on conidia binding than surface topography. This is in agreement with work by Whitehead et al., [50] who demonstrated that Escherichia coli retention to surfaces was mainly affected by the physicochemical properties of the surfaces.
With regards to the surface chemistry, with the exception of the A. pullulans attachment and retention assays, and the A. niger 1988 retention assay, the spore counts were greater on the PVOH surfaces. Atomic force microscopy is a useful instrument that has a number of different applications. Although most well-known for its use to measure the topography of surfaces, an available application on some systems is the use of phase contrast microscopy. Phase contract microscopy is used to detect and quantify changes in composition across the polymer surface to determine the viscoelastic behaviour of the different polymer compositions [55]. An understanding of such surface behaviour is important since surfaces do not always perform as expected [9]. The phase contrast images demonstrated that there were more differences in the frictional forces of the PVAc than the PVOH surfaces. In agreement with this result, the cantilever force measurements demonstrated that there were areas of greater frictional value on the PVAc surface compared to the PVOH surface. Following the adhesion assays, the spores attached in lower numbers to the surface with the more homogeneous surface chemistry. It has been suggested that chemically heterogeneous surfaces may contain a relatively small number of highly adhesive sites which may influence the microbial response to a surface [9], and this was observed in this work. The analysis of areas of chemical heterogeneity is important since they can often contain regions susceptible to microbial biodegradation [56]. It has been previously shown that the intensity of polymer degradation biofouling were determined by influenced by the phase structure [57]. Degradation susceptible regions of polymers regions are believed to range from nano- to micrometers upon exposure to aggressive environments, and such areas can severely impede the protective performance of the surfaces [56]. Thus, in agreement with Ma et al., [9] it may be that it is important that in order to develop surfaces that are relatively non-adhesive to microorganisms, such surfaces should have a highly uniform surface chemistry.
The surface wettabilities demonstrated that the PVAc surface was less wettable than the PVOH surface, whilst the PVOH surface was more wettable and hence demonstrated a greater polarity. Although there was no significant difference in the surface roughness values with the exception of the maximum height, there was a significant difference in the wettabilities of the two surfaces tested in this study; however the difference was only 16°. Such a small difference may be influenced by the uni- and multi directional striations demonstrated on the surface topographies. It has been demonstrated that surfaces with striations will influence contact angle results since the solvents will elongate in the direction of the striations [58]. However, work by Busscher et al. [59] used twelve different commercial polymers after various surface roughening procedures and determined the advancing and receding contact angles of different liquids. They reported that the surface roughening decreased if the contact angles on the smooth surface was lower than 60°. However, surface roughening increased contact angles if the contact angle on the smooth surface was above 86° [59] Further, they found that for contact angles on the smooth surface between 60° and 86°, surface roughening was found not to influence the measured angles, as predicted by the Wenzel equation [59]. Thus, since the PVAc used in this work demonstrated unidirectional lines across the surface whilst the PVOH demonstrated surface striations that were more varied in direction the surface, the wettabilities recorded may have been influenced by the underlying topographical surface features. However, given that the wettabilities of the surfaces used in this study were between 60° and 86°, more work would be needed to determine the extent of this phenomenon on the contact angle measurements. Nonetheless, since the surface wettability results were reinforced by the FTIR results which demonstrated the presence of the OH- groups that are the polar species of the PVOH surface making it more wettable, in this instance it would be suggested that the PVOH was the more wettable surface. Spore germination has been shown to be dependent upon surface wettability characteristics [60]. Studies have shown that spore germination of Phyllosticta ampelicida was highest on the most hydrophobic of surfaces [61,62,63]. Thus, understanding the interaction between critical surface components and fungal conidia will enhance the development of materials which exhibit reduced fungal binding and which may subsequently lower levels of biodegradation.
Within this work, three differently shaped, sized and wettability of spores were used. In terms of size, A. niger 1957 < A. niger 1988 < A. pullulans. Following the attachment assays, the largest spore, A. pullulans was attached in greater numbers to the PVAc surface. However, on the PVOH surfaces, the smallest spores attached in the greatest numbers for all the assays. Although work by Gumargalieva et al. [64] demonstrated that for fungal conidia, adhesion to the surfaces was significantly influenced by large fungal spores, but adhesion was much less influenced by smaller fungal spores, the opposite trend was observed in this work, on the PVOH surface following all the assays. This suggests that the influence of spore size on conidia binding may also be dependent on the type of surface or experimental assay used.
In this study, the determination of the wettability of the conidia was carried out. Such solvent assays, measure the interplay of electrostatic interactions, hydrophobic van der Waals, and various short-range interactions, rather than the wettability of the conidida [65], but for resolution, the term wettability was used to describe the differences of the intermolecular forces demonstrated in this work. Fungal spore wettability measured via toluene assays demonstrated that A. pullulans conidia were the most wettable. This result may have occurred since A. pullulans produces extracellular polysaccharides, in particular pullulan and it is the chlamydospores that are particularly important in the production of this material [66]. Pullulan is a polysaccharide composed of maltotriose units, and pullulan released by spores has been shown to contribute to the adhesion of spores to surfaces [67]. In contrast, the A. niger spp. were more non wettable. Aspergillus spp. spores contain hydrophobins, which are small, amphiphilic, filamentous fungal proteins that self-assemble at water–air interphases [68] In Aspergillus spp. the hydrophobins are formed into rodlet structures on the conidiospore surface and contribute to the spores hydrophobicity, and each fungus is thought to contain a number of different hydrophobins [69]. The rodlet layer composed of hydrophobic Rod A protein and DHN-melanin [23]. The melanin is a pigment which allows the conidia to withstand extremes of condition such as high temperature, but it also allows the conidia to escape recognition by the host immune system [70]. Thus, the chemistry of the spore surface will influence the wettability of the conidia.
The use of the determination of microbial binding to surfaces has been investigated by a number of workers [2, 71]. However, the pattern of conidia binding on a surface is a different phenomenon to the number of spores bound to a surface and the two need to be separated. Following visualisation of the spores bound to the surfaces, it was demonstrated that following the attachment assay, the A. niger 1988 was more homogeneously spread out on the surfaces than the A. niger 1957, which suggests an effect of surface chemistry, or spore shape on conidial attachment. The A. pullulans was clearly clumped together on the more non-wettable PVAc surface, suggesting that the surface wettability had a greater effect on the pattern of A. pullulans attachment. The adhesion assay demonstrated that the pattern of retention of the conidia on the surface was well distributed for the fungal species. The adhesion assays demonstrated that on the PVAc surface the A. niger 1957 and A. niger 1988 spores that could be seen were paired together but on the PVOH surfaces, the A. niger spp. spores looked more homogeneous in spread. The A. pullulans looked to be well spread out on the PVAc surface, but was in small clumps on the PVOH surface. These results suggest that the inclusion of the washing step distributed the spores in a different pattern compared to the attachment assay. The results of the retention assay demonstrated that the inclusion of the washing step resulted in all the spores being more homogeneously distributed across the surface. Thus, the influence of the washing step, and the effect it has on the influence of the effect of surface properties on cell binding requires further attention. The distribution pattern on the conidia on the surfaces may suggest that the influence of spore and surface properties needs to be considered for each type of assay.
The results from the spore assays suggest that the different experimental assays exerted different influences on the surface and spore binding. Following the attachment assays, it was evidenced that the spores were attracted to the surfaces of opposing wettabilities, thus suggesting a combined effect of surface and spore chemical and physicochemical interactions. However, following the adhesion assays, all the spores were adhered in greater numbers to the more wettable PVOH surface, suggesting that the immediate inclusion of a washing step altered the hydration dynamics between the surface and the fungal conidia. The retention assay which also contained a washing step, but was carried out over a longer time period resulted in a similar scenario that followed the attachment assay whereby the round A. niger 1957. and A. pullulans spores attached in greater numbers to the surfaces of opposite wettabilities. However, in this case the same result was not observed for the A. niger 1988 species, suggesting in the case of the retention assay, that spore shape influenced the results. Previous literature has shown that some fungal conidia are highly hydrophobic [72,73,74]. An increase in surface wettability is often associated with an increase in surface roughness [75], although this was not demonstrated in this work. Conversely, the more hydrophilic A. pullulans spores revealed greater adherence toward the hydrophobic PVAc surface. It has previously been determined that the adhesion of A. pullulans was related to the presence and secretion of specific cell surface macromolecules, such as, pullulan and uronic acid based polymers [7]. This effect could also be due to electrostatic interactions which principally control adhesion of A. pullulans to plasticized polyvinyl chloride [33]. It has previously been suggested that the balance between attractive and repulsive interactions can be altered due to differing experimental conditions and forces such as hydrodynamic forces [76]. However, determining and quantifying the effect of such forces at the cell:surface interface is difficult [77]. Alongside the assay type, it is well known that cell surfaces are structurally and chemically complex [76]. Such factors add layers of complexity so that understanding results becomes more multifaceted [78]. In agreement with this work, using four the spores from Geobacillus spp., it was demonstrated that it was difficult to determine a relationship between individual spore adherence and physicochemical interactions [8]. They concluded that surface modifications may be made to reduce the attachment of different strains of Geobacillus spores, but the surface modifications would not be effective for all the spore types, and hence individual surface modifications would be needed to control the particular strains of concern [8]. However, determining the role of different conditions which affect conidia binding on the surfaces will increase the understanding of cell:surface interactions, enabling the development of novel surfaces for targeted commercial and industrial applications.
The different assays used, were selected to represent different applied and environmental situations. For example, the attachment assay was indicative of a surface that becomes fouled with spores carried in water, but is not followed by a washing step, such as might occur from rain on a surface. The adhesion assay, resembles a situation that may occur in a factory environment, whereby a surface is fouled then washed immediately. The retention assay was more indicative of a surface whereby the surface is held for a length of time in a solution. The results from this work suggest that when trying to elucidate the interactions of surface and fungal properties on one another that the assay used clearly influences the pattern of conida binding, and thus the effects of attachment, adhesion and retention on microbial binding should be considered independently. The choice of assay to use and the most effective surface to use should be considered with respect to the final application and environment in which the surface is to be used. In this work, in environments that were indicative of a attachment or retention assay a PVAc surface would reduce the number of A. niger spp. spores whilst a PVOH surface would reduce the number of A. pullulans spores. However, in an environment similar to a adhesion assay, a PVAc surface would be most beneficial to reduce spore retention.
Conclusion
This study highlights that the experimental assay used is of paramount importance when determining the effect of surface properties in fungal spore binding. The addition of a washing step may have disrupted the hydration forces thus ensuing these results. Although surface features or topography did not influence the pattern of spore retention, the surface and spore chemistry and wettability, alongside the assay type most influence condidal:surface binding. Thus, the use of the correct methodology that reflects the environment in which the surface is to be used is important in order to accurately inform hygienic surface development.
References
Soncini G, Franchetti SMM, Marconato JC (2003) Braz J Microbiol 34:105
Whitehead KA, Deisenroth T, Preuss A, Liauw CM, Verran J (2011) Colloids Surf B 82:483
Magnin A, Hoornaert L, Pollet E, Laurichesse S, Phalip V, Averous L (2019) Microb Biotechnol 12:544
Iyyappan J, Baskar G, Bharathiraja B, Saravanathamizhan R (2018) Bioresour Technol 269:393
John MJ (2017) Environmental degradation in biocomposites. Biocomposites for High-Performance Applications. Elsevier, Amsterdam
Cappitelli F, Sorlini C (2008) Appl Environ Microbiol 74:564
Pouliot JM, Walton I, Nolen-Parkhouse M, Abu-Lail LI, Camesano TA (2005) Biomacromol 6:1122
Seale RB, Flint SH, McQuillan AJ, Bremer PJ (2008) Appl Environ Microbiol 74:731
Ma H, Winslow CJ, Logan BE (2008) Colloids Surf B 62:232
Nomura T, Minamiura M, Fukamachi K, Yumiyama S, Kondo A, Naito M (2018) Adv Powder Technol 29:909
Busscher HJ, Norde W, Sharma PK, van der Mei HC (2010) Curr Opin Colloids Interface Sci 15:510
Khan U, May P, Porwal H, Nawaz K, Coleman JN (2013) ACS Appl Mater Int 5:1423
Shit SC, Shah PM, (2014) J Polymers 427259
Razzak MT, Darwis D (2001) Rad Phys Chem 62:107
Geisari N, Kalnins M (2016) IOP Conference series: materials science and engineering. IOP Publishing 012009.
Gaaz T, Sulong A, Akhtar M, Kadhum A, Mohamad A, Al-Amiery A (2015) Molecules 20:22833
Premraj R, Doble M (2005) Indian J Biotechnol 4:186
Teertstra WR, Tegelaar M, Dijksterhuis J, Golovina EA, Ohm RA, Wösten HA (2017) Fungal Gen Biol 98:61
Frisvad JC, Moller LLH, Larsen TO, Kumar R, Arnau J (2018) Appl Microbiol Biotech 102:9481
Panackal AA, Li H, Kontoyiannis DP, Mori M, Perego CA, Boeckh M et al (2010) Clin Infect Dis 50:1588
Anderson J, Smith J (1971) J Gen Microbiol 69:185
Baker SE (2006) Med Mycol 44:S17
Challa S (2018) Curr Fungal Infect Rep 12:23
Marr KA, Carter RA, Crippa F, Wald A, Corey L (2002) Clin Infect Dis 34:909
Steinbach WJ, Benjamin DK, Kontoyiannis DP, Perfect JR, Lutsar I, Marr KA et al (2004) Clin Infect Dis 39:192
Balajee SA, Weaver M, Imhof A, Gribskov J, Marr KA (2004) Antimicrob Agents Chemother 48:1197
Chamilos G, Kontoyiannis DP (2006) Med Mycol 44:163
Chi Z, Wang F, Chi Z, Yue L, Liu G, Zhang T (2009) Appl Microbiol Biotechnol 82:793
Zhang DP, Spadaro D, Garibaldi A, Gullino ML (2010) Biol Control 54:172
Pechak DG, Crang RE (1977) Mycologia 69:792
Catley BJ (1980) J Gen Microbiol 120:265
Kovaltsova SV, Azkharov IA, Levitin MM (1970) Tsitologiya 12:233
Webb JS, Van der Mei HC, Nixon M, Eastwood IM, Greenhalgh M, Read SJ, Robson GD, Handley PS (1999) Appl Environ Microbiol 65:3575
Arvanitidou M, Kanellou K, Constantinides T, Katsouyannopoulos V (1999) Lett Appl Microbiol 29:81
Ducker W, Senden T, Pashley R (1991) Nature 353:239
Bowen WR, Lovitt RW, Wright CJ (2000) Colloids Surf A 173:205
Van Oss C, Giese R (1995) Clays Clay Miner 43:474
Van Oss CJ, Chaudhury MK, Good RJ (1988) Chem Rev 88:927
Mozes N, Rouxhet PG (1987) J Microbiol Meth 6:99
Jeffs LB, Khachatourians GG (1997) Can J Microbiol 43:23
Rajab FH, Liauw CM, Benson PS, Li L, Whitehead KA (2018) Food Bioprod Process 109:29
Shimao M (2001) Curr Opin Biotechnol 12:242
Rajab FH, Liauw CM, Benson PS, Li L, Whitehead KA (2017) Colloids Surf B 160:688
Cai C-X, Xu J, Deng N-F, Dong X-W, Tang H, Liang Y, Fan X-W, Li Y-Z (2016) Sci Rep 6:36546
Pathak VM (2017) Bioresour Bioprocess 4:15
Wickens D, Lynch S, West G, Kelly P, Verran J, Whitehead KA (2014) J Microbiol Meth 104:101
Whitehead KA, Verran J (2007) Inter Biodeterior Biodegrad 60:74
Medilanski E, Kaufmann K, Wick LY, Wanner O, Harms H (2002) Biofouling 18:193
Whitehead KA, Colligon J, Verran J (2005) Colloids Surf B 41:129
Whitehead KA, Olivier S, Benson PS, Arneborg N, Verran J, Kelly P (2015) Int J Food Microbiol 197:92
Tetlow LA, Lynch S, Whitehead KA (2017) Food Bioprod Process 102:332
Kang S-H, Lee H-J, Hong S-H, Kim K-H, Kwon T-Y (2013) Acta Odontol Scand 71:241
Giraldez MJ, Resua CG, Lira M, Oliveira MECR, Magariños B, Toranzo AE, Yebra-Pimentel E (2010) Optom Vis Sci 87:E426
Whitehead KA, Verran J (2006) Food Bioprod Process 84:253
Scott WW, Bhushan B (2003) Ultramicroscopy 97:151
Raghavan D, Gu X, Nguyen T, Van Landingham M, Karim A (2000) Macromolecules 33:2573
Varyan IA, Mastalygina EE, Kolesnikova NN, Popov AA (2018) AIP Conf Proc 1981:020119
Bikerman JJ (1950) J Colloid Sci 5:349
Busscher HJ, Van Pelt AWJ, De Boer P, De Jong HP, Arends J (1984) Colloids Surf 9:319
Chaky J, Anderson K, Moss M, Vaillancourt L (2001) Phytopathology 91:558
Kuo K, Hoch H (1996) Fungal Gen Biol 20:18
Shaw B, Hoch H (1999) Mycol Res 103:915
Shaw BD, Hoch H (2000) Fungal Gen Biol 31:43
Gumargalieva KZ, Kalinina IG, Semenov SA, Zaikov GE (2008) Mol Cryst Liq Cryst 486:1
van der Mei HC, van de Belt-Gritter B, Busscher HJ (1999) Colloids Surf B 5:117
Simon L, Caye-Vaugien C, Bouchonneau M (1993) J Gen Microbiol 139:979
Bardage SL, Bjurman J (1998) Can J Microbiol 44:954
Grunbacher A, Throm T, Seidel C, Gutt B, Rohrig J, Strunk T, Vincze P, Walhei S, Schimmel T, Wenzel W, Fischer R (2014) PLoS ONE 9:e94546
Linder MB, Szilvay GR, Nakari-Setala T, Penttila ME (2005) FEMS Microbiol Rev 29:877
Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR et al (2009) Nature 460:1117
Kalinina IG, Gumargalieva KZ, Semenov SA, Kazarin VV, Semenov SA (2018) Russ J Phys Chem B 12:155
Luke B, Faull J, Bateman R (2015) Biocontrol Sci Technol 25:383
Dague E, Alsteens D, Latgé J-P, Dufrêne YF (2008) Biophys J 94:656
Shan LT, Wang ZL, Ying SH, Feng MG (2010) Mycopathologia 169:483
Arnold M (2010) PRA's 7th International Woodcoatings Congress
Bos R, Van der Mei HC, Busscher HJ (1999) FEMS Microbiol Rev 23:179
Kurihara K (2019) Pure Appl Chem 91:707
Handley, P.S. (1990) Biofouling 2003A239
Funding
This work was funded by BASF Inc. (previously Ciba Specialty Chemicals Inc. Germany).
Author information
Authors and Affiliations
Contributions
Conceptualization:; Methodology: KAW, CL and JW-N; Formal analysis and investigation: KAWW; Writing—original draft preparation: KAW; Writing—review and editing: CM L, AJS, JAB, JSTW-N, TD, AP, JV and KAW; Funding acquisition: KAW and JV; Resources: TD, AP; Supervision: TD, AP, JV and KAW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liauw, C.M., Slate, A.J., Butler, J.A. et al. The Effect of Surface Hydrophobicity on the Attachment of Fungal Conidia to Substrates of Polyvinyl Acetate and Polyvinyl Alcohol. J Polym Environ 28, 1450–1464 (2020). https://doi.org/10.1007/s10924-020-01693-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01693-z