Abstract
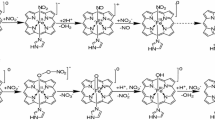
Myoglobin (Mb), generally taken as the molecular model of monomeric globular heme-proteins, is devoted: (i) to act as an intracellular oxygen reservoir, (ii) to transport oxygen from the sarcolemma to the mitochondria of vertebrate heart and red muscle cells, and (iii) to act as a scavenger of nitrogen and oxygen reactive species protecting mitochondrial respiration. Here, the first evidence of ·NO inhibition of ferric Mb- (Mb(III)) mediated detoxification of peroxynitrite is reported, at pH 7.2 and 20.0 °C. ·NO binds to Mb(III) with a simple equilibrium; the value of the second-order rate constant for Mb(III) nitrosylation (i.e., ·NOkon) is (6.8 ± 0.7) × 104 M−1 s−1 and the value of the first-order rate constant for Mb(III)-NO denitrosylation (i.e., ·NOkoff) is 3.1 ± 0.3 s−1. The calculated value of the dissociation equilibrium constant for Mb(III)-NO complex formation (i.e., ·NOkoff/·NOkon = (4.6 ± 0.7) × 10−5 M) is virtually the same as that directly measured (i.e., ·NOK = (3.8 ± 0.5) × 10−5 M). In the absence of ·NO, Mb(III) catalyzes the conversion of peroxynitrite to NO3−, the value of the second-order rate constant (i.e., Pkon) being (1.9 ± 0.2) × 104 M−1 s−1. However, in the presence of ·NO, Mb(III)-mediated detoxification of peroxynitrite is only partially inhibited, underlying the possibility that also Mb(III)-NO is able to catalyze the peroxynitrite isomerization, though with a reduced rate (Pkon* = (2.8 ± 0.3) × 103 M−1 s−1). These data expand the multiple roles of ·NO in modulating heme-protein actions, envisaging a delicate balancing between peroxynitrite and ·NO, which is modulated through the relative amount of Mb(III) and Mb(III)-NO.
Graphic abstract






Similar content being viewed by others
Abbreviations
- DFT:
-
Density functional theory
- Hb:
-
Hemoglobin
- Hb(III):
-
Ferric Hb
- Hb(IV)=O:
-
Ferryl-Hb
- Hb(II):
-
Ferrous Hb
- Hb(II)-CO:
-
Carbonylated Hb(II)
- Hb(II)-NO:
-
Nitrosylated Hb(II)
- Hb(II)-O2 :
-
Oxygenated Hb(II)
- Mb:
-
Myoglobin
- Mb(III):
-
Ferric Mb
- Mb(IV)=O:
-
Ferryl-Mb
- Mb(III)-NO:
-
Nitrosylated Mb(III)
- Mb(II)-CO:
-
Carbonylated Mb(II)
- Mb(II)-NO:
-
Nitrosylated Mb(II)
- Mb(II):
-
Ferrous Mb
- Mb(II)-O2 :
-
Oxygenated Mb(II)
- MI− :
-
4-Methyl-1H-imidazolate
- HMI:
-
4-Methyl-1H-imidazol
- ONOO− :
-
Peroxynitrite anion
- ONOOH:
-
Peroxynitrous acid
References
Goldstein S, Lind J, Merényi G (2005) Chem Rev 105:2457–2470
Herold S, Exner M, Nauser T (2001) Biochemistry 40:3385–3395
Herold S, Shivashankar K (2003) Biochemistry 42:14036–14046
Herold S, Kalinga S, Matsui T, Watanabe Y (2004) J Am Chem Soc 126:6945–6955
Møller JKS, Skibstedm LH (2004) Chem Eur J 10:2291–2300
Exner M, Herold S (2000) Chem Res Toxicol 13:287–293
Bunn HF, Forget BG (1986) Hemoglobin: molecular, genetic and clinical aspects. WB Sauders Co, Philadelphia
Brunori M (2001) Trends Biochem Sci 26:21–23
Brunori M (2001) Trends Biochem Sci 26:209–210
Bannister JV, Bannister WH, Rotilio G (1987) CRC Crit Rev Biochem 22:111–180
Halliwell B (1978) Cell Biol Int Rep 2:113–128
Beckman JS, Koppenol WH (1996) Am J Physiol 271:C1424–C1437
Herold S, Fago A (2005) Comp Biochem Physiol A Mol Integr Physiol 142:124–129
Goldstein S, Merényi G (2008) Methods Enzymol 436:49–61
Ascenzi P, di Masi A, Sciorati C, Clementi E (2010) Biofactors 36:264–273
Radi R, Beckman JS, Bush KM, Freeman BA (2015) J Biol Chem 290:30726–30727
Bartesaghi S, Radi R (2018) Redox Biol 14:618–625
Herold S (2001) Rehmann F-JK. J Biol Inorg Chem 6:543–555
Herold S (2003) Rehmann F-JK. Free Radical Biol Med 34:531–545
Herold S (2004) Inorg Chem 43:3783–3785
Herold S (2006) Inorg Chem 45:6933–6943
Ascenzi P, Ciaccio C (2007) Coletta M. Biochem Biophys Res Commun 363:931–936
Hoshino M, Maeda M, Konishi R, Seki H, Ford PC (1996) J Am Chem Soc 118:5702–5707
Barbosa RM, Lopes Jesus AJ, Santos RM, Pereira CL, Marques CF, Rocha BS, Ferreira NR, Ledo A, Laranjin J (2011) Global J Anal Chem 2:272–284
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. North Holland Publishing Co, Amsterdam
Ascenzi P, Brunori M, Pennesi G, Ercolani C, Monacelli F (1987) J Chem Soc Dalton Trans 369–371
Mehl M, Daiber A, Herold S, Shoun H, Ullrich V (1999) Nitric Oxide 3:142–152
Shimanovich R, Groves JT (2001) Arch Biochem Biophys 387:307–317
Jensen MP, Riley DP (2002) Inorg Chem 41:4788–4797
Ascenzi P, di Masi A, Coletta M, Ciaccio C, Fanali G, Nicoletti FP, Smulevich G, Fasano M (2009) J Biol Chem 284:31006–31017
Ascenzi P, Bolli A, Gullotta F, Fanali G, Fasano M (2010) IUBMB Life 62:776–780
Ascenzi P, Ciaccio C, Sinibaldi F, Santucci R, Coletta M (2011) Biochem Biophys Res Commun 404:190–194
Ascenzi P, Ciaccio C, Sinibaldi F, Santucci R, Coletta M (2011) Biochem Biophys Res Commun 415:463–467
Ascenzi P, Bolli A, di Masi A, Tundo GR, Fanali G, Coletta M, Fasano M (2011) J Biol Inorg Chem 16:97–108
di Masi A, Gullotta F, Bolli A, Fanali G, Fasano M, Ascenzi P (2011) FEBS J 278:654–662. FEBS J 278:4166 (Erratum)
Ascenzi P, Coletta A, Cao Y, Trezza V, Leboffe L, Fanali G, Fasano M, Pesce A, Ciaccio C, Marini S, Coletta M (2013) PLoS One 8:e69762
Coppola D, Giordano D, Tinajero-Trejo M, di Prisco G, Ascenzi P, Poole RK, Verde C (2013) Biochim Biophys Acta 1834:1923–1931
Ascenzi P, Leboffe L, Pesce A, Ciaccio C, Sbardella D, Bolognesi M, Coletta M (2014) PLoS One 9:e95391
Ascenzi P, Leboffe L, Santucci R, Coletta M (2015) J Inorg Biochem 144:56–61
Ascenzi P, Pesce A (2017) J Biol Inorg Chem 22:1141–1150
Ascenzi P, Coletta M (2018) J Phys Chem B 122:11100–11107
De Simone G, di Masi A, Polticelli F, Ascenzi P (2018) FEBS Open Bio 8:2002–2010
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision B.01. Gaussian Inc, Wallingford
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Phys Rev Lett 91:3–6
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Lanucara F, Chiavarino B, Crestoni ME, Scuderi D, Sinha RK, Maitre P (2011) Fornarini S 50:4445–4452
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3093
Merényi G, Lind J (1998) Chem Res Toxicol 11:243–246
Di Muzio E, Polticelli F, Trezza V, Fanali G, Fasano M, Ascenzi P (2014) Arch Biochem Biophys 560:100–112
Wireko FC, Abraham D (1992) J Protein Eng 5:3–5
Lawson DM, Stevenson CE, Andrew CR, Eady RR (2000) EMBO J 19:5661–5671
Barbieri S, Murphy LM, Sawers RG, Eady RR, Hasnain SS (2008) J Biol Inorg Chem 13:531–540
Ascenzi P, Santucci R, Coletta M, Polticelli F (2010) Biophys Chem 152:21–27
Silkstone G, Kapetanaki SM, Husu I, Vos MH, Wilson MT (2010) J Biol Chem 285:19785–19792
Giacometti GM, Ascenzi P, Bolognesi M, Brunori M (1981) J Mol Biol 146:363–374
Coletta M, Ascenzi P, Traylor TG, Brunori M (1985) J Biol Chem 260:4151–4155
Ascenzi P, Coletta M, Desideri A, Brunori M (1985) Biochim Biophys Acta 829:299–302
Ascenzi P, Condó SG, Bellelli A, Barra D, Bannister WH, Giardina B, Brunori M (1984) Biochim Biophys Acta 788:281–289
Pennesi G, Ercolani C, Rossi G, Ascenzi P, Brunori M, Monacelli F (1995) J Chem Soc Dalton Trans 1113–1118
Buxton GV (1987) Radiation Chemistry. Principles and Applications. In: Farhataziz, Rodgers MAJ (eds) Verlag Chemie Publishers, Weinheim
Spinks JWT, Woods RJ (1990) An introduction to radiation chemistry, 3rd edn. Wiley-Interscience publication, New York
Conti E, Moser C, Rizzi M, Mattevi A, Lionetti C, Coda A, Ascenzi P, Brunori M, Bolognesi M (1993) J Mol Biol 233(3):498–508
Leci E, Brancaccio A, Cutruzzolà F, Allocatelli CT, Tarricone C, Bolognesi M, Desideri A, Ascenzi P (1995) FEBS Lett 357:227–229
Aime S, Fasano M, Paoletti S, Cutruzzolà F, Desideri A, Bolognesi M, Rizzi M, Ascenzi P (1996) Biophys J 70:482–488
Maurus R, Bogumil R, Nguyen NT, Mauk AG, Brayer G (1998) Biochem J 332:67–74
McQuarters AB, Kampf JW, Alp EE, Hu M, Zhao J, Lehnert N (2017) J Am Chem Soc 56:10513–10528
Sharma SK, Schaefer AW, Hyeongtaek L, Matsumura H, Moenne-Loccoz P, Hedman B, Hodgson KO, Solomon EI, Karlin KD (2017) J Am Chem Soc 139:17421–17430
Wittenberg BA, Wittenberg JB (1989) Annu Rev Physiol 51:857–878
Flögel U, Merx MW, Godecke A, Decking UK, Schrader J (2001) J Proc Natl Acad Sci USA 98:735–740
Herold S, Boccini F (2006) Inorg Chem 45:6933–6943
Herold S, Fago A, Weber RE, Dewilde S, Moens L (2004) J Biol Chem 279:22841–22847
Herold S, Puppo A (2005) J Biol Inorg Chem 10:946–957
Ascenzi P, Bocedi A, Antonini G, Bolognesi M, Fasano M (2007) FEBS J 274:551–562
Acknowledgements
The grant of Excellence Departments, MIUR-Italy (Articolo 1, Commi 314-337, Legge 232/2016) is gratefully acknowledged. C. P.-I. thanks Centro de Supercomputación de Galicia (CESGA) for providing the computer facilities (A Coruña, Galicia, Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ascenzi, P., De Simone, G., Tundo, G.R. et al. Ferric nitrosylated myoglobin catalyzes peroxynitrite scavenging. J Biol Inorg Chem 25, 361–370 (2020). https://doi.org/10.1007/s00775-020-01767-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01767-2