Abstract
Summary
Loss of bone mineral density and skeletal muscle area are linked in lung transplant patients. This loss is greater in patients with restrictive compared with obstructive lung diseases.
Introduction
Sarcopenia and osteoporosis are associated with aging and chronic illnesses and may be linked in patients with advanced lung disease. Pectoralis muscle index (PMI) quantitated on computed tomography (CT) of the chest can be used to measure skeletal muscle mass. This study aimed to determine the relationship of PMI to clinical parameters including bone mineral density (BMD) in candidates for lung transplantation.
Methods
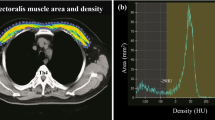
A retrospective review of transplant candidates at a single center was performed. Demographic, anthropomorphic, and clinical data were recorded. Pectoralis muscle area (PMA) was determined on an axial slice from a chest CT. PMI was calculated as the PMA divided by height squared. BMD was obtained from routine dual-energy X-ray absorptiometry (DXA) scan.
Results
In 226 included patients, mean PMI was 8.2 ± 3.0 cm2/m2 in males and 6.1 ± 2.1 cm2/m2 in females. Osteopenia was present in 44.4%, and 23.2% of patients had osteoporosis. Patients with obstructive lung disease had lower body mass index (22.0 ± 4.9 versus 27.9 ± 4.9 kg/m2, p < 0.001), PMI (6.0 ± 2.3 versus 8.2 ± 2.8 cm2/m2, p < 0.001), and BMD (− 2.3 ± 1.1 versus − 1.3 ± 1.1, p < 0.001) compared with patients with restrictive lung disease. PMI was a significant predictor of BMD (β = 0.16, p < 0.001).
Conclusion
The association between muscle area and BMD in lung transplant candidates suggests that similar mechanisms may underlie the development of both. Differences in PMI and BMD in patients with obstructive versus restrictive lung disease may result from differences in respiratory physiology or disease processes.

Similar content being viewed by others
References
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Gillette-Guyonnet S, Nourhashemi F, Andrieu S, Cantet C, Albarede JL, Vellas B, Grandjean H (2003) Body composition in French women 75+ years of age: the EPIDOS study. Mech Ageing Dev 124:311–316
Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, Sergi G, Bosello O, Zamboni M (2004) Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord 28:234–241
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Miller MD, Crotty M, Giles LC, Bannerman E, Whitehead C, Cobiac L, Daniels LA, Andrews G (2002) Corrected arm muscle area: an independent predictor of long-term mortality in community-dwelling older adults? J Am Geriatr Soc 50:1272–1277
Wannamethee SG, Shaper AG, Lennon L, Whincup PH (2007) Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 86:1339–1346
Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF (2005) Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 82:53–59
Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F (2002) Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166:809–813
McDonald ML, Diaz AA, Ross JC et al (2014) Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 11:326–334
Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, Dekhuijzen PN, Franssen F, Gayan-Ramirez G, Gea J, Gosker HR, Gosselink R, Hayot M, Hussain SN, Janssens W, Polkey MI, Roca J, Saey D, Schols AM, Spruit MA, Steiner M, Taivassalo T, Troosters T, Vogiatzis I, Wagner PD, ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD (2014) An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 189:e15–e62
Barreiro E, Sznajder JI, Nader GA, Budinger GR (2015) Muscle dysfunction in patients with lung diseases: a growing epidemic. Am J Respir Crit Care Med 191:616–619
Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J (2015) The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult lung and heart-lung transplantation report--2015; focus theme: early graft failure. J Heart Lung Transplant 34:1264–1277
Tschopp O, Boehler A, Speich R, Weder W, Seifert B, Russi EW, Schmid C (2002) Osteoporosis before lung transplantation: association with low body mass index, but not with underlying disease. Am J Transplant 2:167–172
Calella P, Valerio G, Brodlie M, Donini LM, Siervo M (2018) Cystic fibrosis, body composition, and health outcomes: a systematic review. Nutrition 55-56:131–139
Caplan-Shaw CE, Arcasoy SM, Shane E, Lederer DJ, Wilt JS, O'Shea MK, Addesso V, Sonett JR, Kawut SM (2006) Osteoporosis in diffuse parenchymal lung disease. Chest 129:140–146
Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130
Hwang JA, Kim YS, Leem AY, Park MS, Kim SK, Chang J, Jung JY (2017) Clinical implications of sarcopenia on decreased bone density in men with COPD. Chest 151:1018–1027
Laboratories ATSCoPSfCPF (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–117
van Vugt JL, Levolger S, Gharbharan A, Koek M, Niessen WJ, Burger JW, Willemsen SP, de Bruin RW, JN IJ (2017) A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 8:285–297
Hoang V, Li GW, Kao CC, Dronavalli G, Parulekar AD (2017) Determinants of pre-transplantation pectoralis muscle area (PMA) and post-transplantation change in PMA in lung transplant recipients. Clin Transpl 31
Lee S, Paik HC, Haam SJ, Lee CY, Nam KS, Jung HS, Do YW, Shu JW, Lee JG (2016) Sarcopenia of thoracic muscle mass is not a risk factor for survival in lung transplant recipients. J Thorac Dis 8:2011–2017
Rozenberg D, Mathur S, Herridge M, Goldstein R, Schmidt H, Chowdhury NA, Mendes P, Singer LG (2017) Thoracic muscle cross-sectional area is associated with hospital length of stay post lung transplantation: a retrospective cohort study. Transpl Int 30:713–724
Biskobing DM (2002) COPD and osteoporosis. Chest 121:609–620
Hardin DS, Arumugam R, Seilheimer DK, LeBlanc A, Ellis KJ (2001) Normal bone mineral density in cystic fibrosis. Arch Dis Child 84:363–368
Kim YS, Kim EY, Kang SM, Ahn HK, Kim HS (2017) Single cross-sectional area of pectoralis muscle by computed tomography - correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin Physiol Funct Imaging 37:507–511
Diaz AA, Martinez CH, Harmouche R et al (2018) Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir Res 19:62
Hirschfeld HP, Kinsella R, Duque G (2017) Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 28:2781–2790
Singer JP, Diamond JM, Anderson MR et al (2018) Frailty phenotypes and mortality after lung transplantation: a prospective cohort study. Am J Transplant 18:1995–2004
Rozenberg D, Mathur S, Wickerson L, Chowdhury NA, Singer LG (2018) Frailty and clinical benefits with lung transplantation. J Heart Lung Transplant 37:1245–1253
Venado A, McCulloch C, Greenland JR, Katz P, Soong A, Shrestha P, Hays S, Golden J, Shah R, Leard LE, Kleinhenz ME, Kukreja J, Zablotska L, Allen IE, Covinsky K, Blanc P, Singer JP (2019) Frailty trajectories in adult lung transplantation: a cohort study. J Heart Lung Transplant 38:699–707
Aris RM, Neuringer IP, Weiner MA, Egan TM, Ontjes D (1996) Severe osteoporosis before and after lung transplantation. Chest 109:1176–1183
Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL, Polkey MI, Man WD (2015) Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 70:213–218
Ionescu AA, Nixon LS, Evans WD, Stone MD, Lewis-Jenkins V, Chatham K, Shale DJ (2000) Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med 162:789–794
Pardo A, Selman M (2016) Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 13(Suppl 5):S417–S421
Kao CC, Hsu JW, Bandi V, Hanania NA, Kheradmand F, Jahoor F (2011) Resting energy expenditure and protein turnover are increased in patients with severe chronic obstructive pulmonary disease. Metabolism 60:1449–1455
Donahoe M, Rogers RM, Wilson DO, Pennock BE (1989) Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 140:385–391
Hsia CC (1999) Cardiopulmonary limitations to exercise in restrictive lung disease. Med Sci Sports Exerc 31:S28–S32
Aliverti A (1985) Macklem PT (2008) The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J Appl Physiol 105:749–751 discussion 755-747
England BK, Chastain JL, Mitch WE (1991) Abnormalities in protein synthesis and degradation induced by extracellular pH in BC3H1 myocytes. Am J Phys 260:C277–C282
Jaitovich A, Angulo M, Lecuona E, Dada LA, Welch LC, Cheng Y, Gusarova G, Ceco E, Liu C, Shigemura M, Barreiro E, Patterson C, Nader GA, Sznajder JI (2015) High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific ring finger protein 1 (MuRF1). J Biol Chem 290:9183–9194
Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, Aliverti A (2010) Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax 65:808–814
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parulekar, A.D., Wang, T., Li, G.W. et al. Pectoralis muscle area is associated with bone mineral density and lung function in lung transplant candidates. Osteoporos Int 31, 1361–1367 (2020). https://doi.org/10.1007/s00198-020-05373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05373-5