Abstract
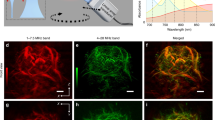
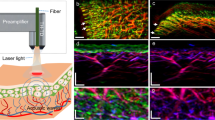
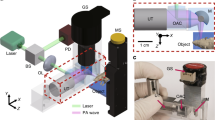
The monitoring of vascular-targeted therapies using magnetic resonance imaging, computed tomography or ultrasound is limited by their insufficient spatial resolution. Here, by taking advantage of the intrinsic optical properties of haemoglobin, we show that raster-scanning optoacoustic mesoscopy (RSOM) provides high-resolution images of the tumour vasculature and of the surrounding tissue, and that the detection of a wide range of ultrasound bandwidths enables the distinction of vessels of differing size, providing detailed insights into the vascular responses to vascular-targeted therapy. Using RSOM to examine the responses to vascular-targeted photodynamic therapy in mice with subcutaneous xenografts, we observed a substantial and immediate occlusion of the tumour vessels followed by haemorrhage within the tissue and the eventual collapse of the entire vasculature. Using dual-wavelength RSOM, which distinguishes oxyhaemoglobin from deoxyhaemoglobin, we observed an increase in oxygenation of the entire tumour volume immediately after the application of the therapy, and a second wave of oxygen reperfusion approximately 24 h thereafter. We also show that RSOM enables the quantification of differences in neoangiogenesis that predict treatment efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data from this study are available within the Article and its Supplementary Information. Raw data for the individual measurements are available on reasonable request.
References
Lin, Z., Zhang, Q. & Luo, W. Angiogenesis inhibitors as therapeutic agents in cancer: challenges and future directions. Eur. J. Pharmacol. 793, 76–81 (2016).
Gunther, J. E. et al. Dynamic diffuse optical tomography for monitoring neoadjuvant chemotherapy in patients with breast cancer. Radiology 287, 778–786 (2018).
Madar-Balakirski, N. et al. Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad. PLoS ONE 5, e10282 (2010).
Goldschmidt, R., Vyacheslav, K. & Scherz, A. Real time laser speckle imaging monitoring vascular targeted photodynamic therapy. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy (eds Kessel, D. H. & Hasan, T.) XXVI (SPIE, 2017).
Gross, S., Gilead, A., Scherz, A., Neeman, M. & Salomon, Y. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat. Med. 9, 1327–1331 (2003).
Fleshker, S., Preise, D., Kalchenko, V., Scherz, A. & Salomon, Y. Prompt assessment of WST11-VTP outcome using luciferase transfected tumors enables second treatment and increase in overall therapeutic rate. Photochem. Photobiol. 84, 1231–1237 (2008).
Cornelis, F. H. et al. Contrast enhanced ultrasound imaging can predict vascular-targeted photodynamic therapy induced tumor necrosis in small animals. Photodiagn. Photodyn. Ther. 20, 165–168 (2017).
Neuschmelting, V. et al. WST11 vascular targeted photodynamic therapy effect monitoring by multispectral optoacoustic tomography (MSOT) in mice. Theranostics 8, 723–734 (2018).
Johnson, S. P., Ogunlade, O., Lythgoe, M. F., Beard, P. & Pedley, R. B. Longitudinal photoacoustic imaging of the pharmacodynamic effect of vascular targeted therapy on tumors. Clin. Cancer Res. 25, 7436–7447 (2019).
Ntziachristos, V. & Razansky, D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem. Rev. 110, 2783–2794 (2010).
Taruttis, A. & Ntziachristos, V. Advances in real-time multispectral optoacoustic imaging and its applications. Nat. Photonics 9, 219–227 (2015).
Aguirre, J. et al. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 1, 0068 (2017).
Omar, M., Schwarz, M., Soliman, D., Symvoulidis, P. & Ntziachristos, V. Pushing the optical imaging limits of cancer with multi-frequency-band raster-scan optoacoustic mesoscopy (RSOM). Neoplasia 17, 208–214 (2015).
Omar, M. et al. Optical imaging of post-embryonic zebrafish using multi orientation raster scan optoacoustic mesoscopy. Light Sci. Appl. 6, e16186 (2017).
Aguirre, J. et al. Broadband mesoscopic optoacoustic tomography reveals skin layers. Opt. Lett. 39, 6297–6300 (2014).
Schwarz, M., Omar, M., Buehler, A., Aguirre, J. & Ntziachristos, V. Implications of ultrasound frequency in optoacoustic mesoscopy of the skin. IEEE Trans. Med. Imaging 34, 672–677 (2015).
Berezhnoi, A. et al. Assessing hyperthermia-induced vasodilation in human skin in vivo using optoacoustic mesoscopy. J. Biophotonics 11, e201700359 (2018).
Schwarz, M., Buehler, A., Aguirre, J. & Ntziachristos, V. Three-dimensional multispectral optoacoustic mesoscopy reveals melanin and blood oxygenation in human skin in vivo. J. Biophotonics 9, 55–60 (2015).
Mazor, O. et al. WST11, a novel water-soluble bacteriochlorophyll derivative; cellular uptake, pharmacokinetics, biodistribution and vascular-targeted photodynamic activity using melanoma tumors as a model. Photochem. Photobiol. 81, 342–351 (2005).
Brandis, A. et al. Novel water-soluble bacteriochlorophyll derivatives for vascular-targeted photodynamic therapy: synthesis, solubility, phototoxicity and the effect of serum proteins. Photochem. Photobiol. 81, 983–993 (2005).
Azzouzi, A. R. et al. TOOKAD® soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J. Urol. 33, 945–953 (2015).
Noweski, A. et al. Medium-term follow-up of vascular-targeted photodynamic therapy of localized prostate cancer using TOOKAD soluble WST-11 (phase II trials). Eur. Urol. Focus 5, 1022–1028 (2018).
Taneja, S. S. et al. Final results of a phase I/II multicenter trial of WST11 vascular targeted photodynamic therapy for hemi-ablation of the prostate in men with unilateral low risk prostate cancer performed in the United States. J. Urol. 196, 1096–1104 (2016).
Ashur, I. et al. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin. J. Phys. Chem. A 113, 8027–8037 (2009).
Durand, R. E. Intermittent blood flow in solid tumours—an under-appreciated source of ‘drug resistance’. Cancer Metastasis Rev. 20, 57–61 (2001).
Morikawa, S. et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 160, 985–1000 (2002).
Rahbari, N. N. et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med. 8, 360ra135 (2016).
Jain, R. K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014).
Hernandez-Agudo, E. et al. Monitoring vascular normalization induced by antiangiogenic treatment with 18F-fluoromisonidazole-PET. Mol. Oncol. 10, 704–718 (2016).
Yang, J. et al. MR imaging biomarkers evaluating vascular normalization window after anti-vessel treatment. Oncotarget 9, 11964–11976 (2018).
Biel, N. M., Lee, J. A., Sorg, B. S. & Siemann, D. W. Limitations of the dorsal skinfold window chamber model in evaluating anti-angiogenic therapy during early phase of angiogenesis. Vasc. Cell 6, 17 (2014).
Kimm, S. Y. et al. Nonthermal ablation by using intravascular oxygen radical generation with WST11: dynamic tissue effects and implications for focal therapy. Radiology 281, 109–118 (2016).
Mallidi, S., Watanabe, K., Timerman, D., Schoenfeld, D. & Hasan, T. Prediction of tumor recurrence and therapy monitoring using ultrasound-guided photoacoustic imaging. Theranostics 5, 289–301 (2015).
Wang, H. W. et al. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 64, 7553–7561 (2004).
Jathoul, A. P. et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photonics 9, 239–246 (2015).
He, H. et al. Capsule optoacoustic endoscopy for esophageal imaging. J. Biophotonics 12, e201800439 (2019).
Haedicke, K. et al. Sonophore labeled RGD: a targeted contrast agent for optoacoustic imaging. Photoacoustics 6, 1–8 (2017).
Caballero, M. A. A. Incorporating Sensor Properties in Optoacoustic Imaging. PhD thesis, Technical Univ. Munich (2013).
Acknowledgements
We thank N. Fan of the MSKCC Molecular Cytology Core Facility for assistance with histology; Y. Romin for support and feedback on image analysis; and D. Yarilin of the MSKCC Molecular Cytology Core Facility for immunohistochemistry. This study was funded by the Thompson Family Foundation (Wade F. B. Thompson grant to J.C., A.S. and J.G.) and the National Cancer Institute (grant no. R01 CA212379 to J.G.). The study was also supported in part by the European grant INNODERM (no. 687866) Horizon 2020. We acknowledge P. Zanzonico and V. Ann Longo of the Small Animal Imaging Core Facility of MSKCC for their support (the Core is funded by the NIH Cancer Centre Support grant no. P30 CA008748).
Author information
Authors and Affiliations
Contributions
K.H. designed and performed all of the experiments, processed and analysed the RSOM data, evaluated histological sections and wrote the manuscript. L.A. performed the VTP experiments in CT26 tumours and provided practical input for study planning and performance. M.O. developed the RSOM system, supplied technical input and performed the dual-wavelength RSOM measurements and analysis. A.B. conducted the dual-wavelength RSOM experiments and analysis. S.R. and M.S. conducted the craniotomy and performed the imaging of the mouse brain. K.N. performed the VTP experiments in bladder tumour models and supported RSOM imaging. H.-T.H. performed the VTP experiments in bladder tumour models and supported histological analysis with C.L.-M. K.K. provided conceptual input and designed the experiments in bladder tumour models. T.R. provided input for the design of the brain experiments. J.C. provided input and designed the VTP experiments. V.N. provided technical input for RSOM imaging and supervised the dual-wavelength measurements. A.S. supervised the VTP experiments, provided conceptual input and designed the experiments. J.G. supervised the study, provided input for all of the experiments and the study concept, and edited the paper.
Corresponding author
Ethics declarations
Competing interests
V.N. is a shareholder in iThera Medical GmbH in Munich, Germany, which produces a commercial version of the monospectral RSOM (not used in this study). A.S. is an inventor of padeliporfin and has a financial interest from licensing fees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables, and the caption for Supplementary Video 1.
Supplementary Video 1
Rotating 3D volume of a CT26 tumour.
Rights and permissions
About this article
Cite this article
Haedicke, K., Agemy, L., Omar, M. et al. High-resolution optoacoustic imaging of tissue responses to vascular-targeted therapies. Nat Biomed Eng 4, 286–297 (2020). https://doi.org/10.1038/s41551-020-0527-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0527-8