Abstract
Purpose
The purpose of this study was to compare dynamic 18F-FGln PET/CT images of healthy subjects and cancer patients and explore the best imaging phase for different cancers.
Methods
Thirteen healthy volunteers and 31 cancer patients separately underwent 18F-FGln and 18F-FDG PET/CT scans within 1 week. The distributions of 18F-FGln and 18F-FDG in the whole body and the tumor avidity were analyzed and compared. The tumor maximum standardized uptake values (SUVmax) and tumor-to-nontumor SUV ratio (SUR) of 18F-FGln/PET at different scan phases were compared.
Results
Compared to the healthy subjects, the cancer patients had lower 18F-FGln activity (SUVmean) in most normal organs, especially in the lung, muscle, spleen, and heart (p < 0.05). Additionally, the FGln-avid tumors did not necessarily manifest as FDG-avid and vice versa. Overall, among the 31 primary malignant lesions confirmed by biopsy or postoperative pathological analysis, 29 showed increased radioactive uptake on all 18F-FGln PET/CT imaging phases. The peak of SUVmax in breast and thyroid cancers was within 10 min, while in lung cancers, the plateau of SUVmax was within 30 min to 60 min. The SURs of lung cancer (p = 0.046) and thyroid cancer (p = 0.794) increased from the early-phase to the late-phase acquisition; however, a significant decrease was observed in the breast lesions (p = 0.022).
Conclusions
18F-FGln images may further supplement the diagnostic ability of 18F-FDG in cancer patients and detect metabolic changes in different tumors. Furthermore, the imaging time for 18F-FGln PET/CT needs to be optimized for different cancer types to improve the contrast resolution of tumors.
Similar content being viewed by others
Introduction
Tumor cells require extra energy and metabolism to rapidly grow and proliferate; in addition to glucose, glutamine may play a role as an alternative source of nutrients for survival [1]. 18F-(2S,4R)4-fluoroglutamine (18F-FGln), an analog of natural glutamine, has been developed and characterized as a promising probe for investigating tumor glutamine flux and metabolism in vivo [2,3,4,5,6]. This molecule was demonstrated to have a relatively high tumor cell uptake and retention in animal models [7,8,9]. Furthermore, 18F-FGln positron emission tomography (PET) was useful in tracking the size of the cellular glutamine pool in breast cancers with different levels of glutaminase (GLS) activity and detecting increases in the size of the cellular glutamine pool induced by GLS inhibitors [10]. The first use of 18F-FGln/PET imaging in human gliomas showed that this modality was capable of differentiating clinically progressing disease from stable gliomas [4]. Further preliminary studies that evaluated 18F-FGln in a small number of different cancer patients suggested that certain tumors (brain metastases, breast, pancreas, renal, neuroendocrine, lung, colon, lymphoma, bile duct, or glioma) could display high 18F-FGln uptake [2, 5]. Furthermore, Zhu et al. reported that 18F-FGln/PET imaging may be a useful tool for assessing reduced bone marrow activity in cancer patients, who may be at risk of myelosuppression after chemotherapy [11].
Compared to 18F-FDG, 18F-FGln was a relatively new imaging probe for studying cancer metabolism. The intrinsic variability of 18F-FGln distribution in normal organs needs to be investigated before the capability of this probe to detect and monitor different types of cancer can be evaluated; head-to-head comparisons between FGln/PET and FDG/PET from healthy volunteers to cancer patients were novel, and the differences in glutamine metabolism between healthy subjects and cancer patients have not been investigated. Therefore, this study aims to further evaluate dynamic imaging with 18F-FGln in healthy patients and cancer patients and to explore the best phase of PET imaging to maximize the tumor-to-nontumor SUV ratio (SUR) in different types of tumors. Herein, we reported the results of dynamic imaging with 18F-FGln in 44 healthy subjects and cancer patients.
Materials and methods
Production of 18F-(2S,4R)4-fluoroglutamine
18F-FGln was produced by the Nuclear Medicine Department of Peking University Cancer Hospital as previously reported [5].
Subjects
This study was approved by the Ethics Committee of Peking University Cancer Hospital (ID. 2017KT38) and was conducted according to the latest guidelines of the Declaration of Helsinki. Written informed consent was obtained from each participant prior to 18F-FGln PET/CT imaging. The inclusion criteria for healthy subjects included the following: older than 18 years, the ability to provide informed written consent, and a medical history without any significant comorbidities (including physical examination, electrocardiogram, hematology, and biochemistry). The inclusion criterion for oncological patients was a specific malignant tumor. The exclusion criteria included the following: liver and renal function dysfunction, and pregnancy or current lactation. Finally, 4 groups of subjects (13 healthy subjects, 8 lung cancer patients, 17 breast cancer patients, and 6 thyroid cancer patients) were enrolled in this study, for a total of 44 subjects. The demographic data of these patients were shown in Table 1 and Supplemental Tables S1, S2, and S3.
Examination procedures
No specific preparation was required for any of the subjects on the day of 18F-FGln/PET scanning. A low-dose CT scan (120 kV, 35 mA, slice 0.6 mm, matrix 512 × 512) was performed before the 18F-FGln injection, and then, a whole-body dynamic PET scan was performed immediately after the intravenous injection of 18F-FGln in all subjects (3.7 Mbq/kg) and continued for approximately 60 min (6 passes); 6 of 13 healthy volunteers also underwent a static whole-body PET/CT scan at 120 min postinjection that continued for approximately 10 min. Dynamic and/or static whole-body PET/CT scans using a Biograph mCT Flow 64 scanner (Siemens, Erlangen, Germany) (120 kV, 146 mAs, slice 3 mm, matrix 200 × 200, full width at half maximum (FWHM) 5 mm, filter: Gaussian, field of view (FOV) 256 (head), 576 (body)), continuously moved the patient bed at a speed of 1.5 mm/s to cover the entire body of each subject (from the top of the skull to the middle of the femur). The scanning protocol was described in Fig. 1. A three-dimensional iterative reconstruction was used for image reconstruction, with CT-based attenuation and scatter correction through standard vendor-based reconstruction. The 18F activities were decay corrected to the time of injection and normalized to the total activity administered. All patients underwent a follow-up 18F-FDG/PET scan on the same imaging system within a week after 18F-FGln/PET imaging, and the scanning parameters were consistent with those for 18F-FGln.
Data analysis
A Siemens workstation (MultiModality Workplace) was used for postprocessing. Two experienced nuclear medicine physicians independently reviewed all studies, and any discordant results were resolved by consensus. To analyze the biodistribution and variability of 18F-FGln, volumes of interest (VOIs) were manually drawn on every section of major organs/tissues within the edges of each organ, while avoiding major blood vessels, as previously described [12]. The following normal organs and tissues were included in the VOI analysis: the brain, parotid glands, thyroid, heart, lungs, liver, gallbladder, pancreas, spleen, kidneys, red marrow, bone, stomach, small intestine, upper and lower colon, uterus, breast, prostate, testis, and muscle (quadriceps femoris). The software automatically obtained the average standardized uptake value (SUVmean) and total activity in the VOIs. The SUV was corrected by the injected dose and the weight of the subject, and the value was also decay corrected. The SUVmean, coefficient of variation (CV), and total activity were used to assess the variability among all healthy subjects for each organ. Representative whole-organ VOIs in the sagittal, coronal, and axial planes were shown in Supplemental Information (Fig. S1). The same parameters were also derived for the spherical/elliptical VOIs drawn in the center of the normal brain, lungs, heart, liver, kidneys, and spleen, which could be potentially more convenient volumes for clinical applications. With this method, the total activity was calculated by multiplying the averge activity by each organ volume, which was obtained by Philips© Pinnacle3 treatment planning software.
For the SUVs within the tumor lesions, an elliptical VOI was placed over each metabolically active lesion that was suspected to be malignant with a 50% threshold isocontour. Compared to the uptake in the adjacent normal tissue, the focal uptake of 18F-FGln/PET in the tumor lesion was considered positive. To compute the tumor-to-nontumor SUV ratio (SUR), we obtained the nontumor SUV from a disease-free area within the surrounding or contralateral normal tissue [5]. The same method was used to obtain the same quantitative data for 18F-FDG with healthy subjects and cancer patients as the controls. Our study was designed to identify the best imaging phase to acquire static 18F-FGln PET/CT images to obtain the highest SUR and the best visual effect for tumors. For this effect, early, middle, and late 18F-FGln PET/CT imaging phases (10 min.pi, 30 min.pi, and 60 min.pi) were analyzed using data derived from 31 patients.
We retrospectively identified breast cancer patients using the Ki-67 immunohistochemistry (IHC) values available in the pathology database of our hospital. Pathological evaluations were performed according to the World Health Organization (WHO) classification standards and the Scarff-Bloom-Richardson grading system [13]. Under high-power magnification, the positive ratios of three areas (high-labeling index area, low-labeling index area, overall representative area) were estimated by two breast pathologists, and then, the average ratio of the three areas was reported as the Ki-67 score. Finally, the IHC-based Ki-67 expression was estimated using the following scoring system, as reported elsewhere: 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, and > 90 [14].
Dosimetry
Dosimetry calculations were performed using the OLINDA/EXM software [15]. The residence time for each organ was calculated from the non-decay-corrected time-activity curve data. A standard body weight (56.9 kg for women and 73.7 kg for men) and standard organ weights were used to standardize the data, as suggested by the OLINDA/EXM software [16].
Statistical analysis
All statistical tests were conducted using IBM SPSS version 19 for Windows (IBM Corp, Armonk, NY, USA). The data were presented as the mean ± SD and the CV for each organ. The Mann-Whitney U test was used to compare the differences between groups. Pearson’s correlation coefficient (r) was used for correlation analysis between continuous variables. A p value less than 0.05 was considered statistically significant.
Results
Dosimetry
For dosimetry calculations, 6 healthy subjects underwent multiple whole-body PET/CT examinations, generating datasets for 7 time points per organ per individual over a period of 130 min. The mean absorbed doses in the organs per unit of administered activity were calculated by the OLINDA/EXM software for the given residence times determined from the measured 18F-FGln distribution in the body, as previously reported [17]. The results of the 18F-FGln radiation dosimetry study were presented in Table 2. The average effective dose per unit administered activity was calculated as 16.90 ± 2.13 μSv/MBq (male) and 21.90 ± 2.97 μSv/MBq (female), which were below the recommended Food and Drug Administration limits for research studies [18]. Moreover, these values were comparable to the previous radiation dosimetry results obtained from 6 Caucasian patients with glioma (effective dose 18.9 μSv/MBq) [4] from the perspective of clinical radiation exposure.
Distribution of 18F-(2S,4R)4-fluoroglutamine in normal organs of healthy subjects
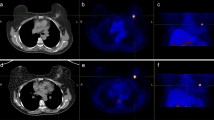
Figure 2 showed a series of maximum-intensity projection images (Fig. 2a) and images from different slices (Fig. 2b, coronal; Fig. 2c, sagittal) of 18F-FGln distribution over time in a representative healthy female subject, and the biodistribution map for several normal organs/tissues was shown in Fig. 3a. Initially, significant uptake was observed in the kidneys, liver, and pancreas (SUVmean > 5). Moderate uptake was observed in the heart, spleen, marrow, uterus, and prostate (2 < SUVmean ≤ 5). There was no significant 18F-FGln uptake in the brain, lung, breast, and muscle (SUVmean ≤ 1). In addition, 18F-FGln/PET showed higher uptake in the trabecular bone of the rib, vertebrae, and pelvis than 18F-FDG (FGln-SUVmean = 3.21 ± 0.51; FDG-SUVmean = 2.11 ± 0.30; p < 0.05), although the 18F-FGln uptake was lower in the appendicular skeleton than in the trabecular bone, with uptake similar to that of 18F-FDG (FGln-SUVmean = 0.94 ± 0.22 and FDG-SUVmean = 0.75 ± 0.26, p > 0.05).
Results of PET/CT imaging studies in one healthy subject. Images were normalized using a same color scale indicated in the bottom row for better visualizing the relative distribution. Top row indicates ending time (min) of whole-body scan. a Decay-corrected anterior maximum-intensity projections (MIP) of PET images at 9, 20, 29, 39, 49, 59, and 131 min (from left to right) after injection of 18F-FGln in a healthy female volunteer (51-year-old). b Whole-body coronal and c mid-sagittal PET/CT sections images of 18F-FGln in the same volunteer
Additionally, the calculated CVs of 20 organs/tissues across the SUVmean values of all healthy volunteers were shown in Supplemental Table S4. The spleen had the lowest variability in 18F-FGln uptake of the studied organs with a CV of 11.4%, whereas the colon had the highest variability (37.6%). The CV of the liver was 14.70%; for reference, the CV of the liver with 18F-FDG was 13.35%. The CV of 18F-FGln uptake in the breast was 13.4%, which was lower than that of 18F-FDG PET uptake in the breast (40.12%); although the density of breast tissue could affect the SUVmean of 18F-FGln, the fluctuation was less than 1.01. In addition, the variabilities between all paired organs were similar (p > 0.05) (Fig. 3b), and the measured radioactivity concentrations in different organs from the whole-body scans did not significantly differ between men and women (p > 0.05).
Although the whole-organ VOI may be the most reliable method for determining the average uptake in normal organs, mapping such VOIs was time consuming and unlikely to be performed in a busy clinical workflow. We investigated the ability of a simple spherical/elliptical VOI placed within a visually normal organ to represent the activity in the whole-organ VOI. The SUVmean and total activity calculated by the spherical/elliptical VOIs were similar with the whole-organ VOI uptake values according to the Mann-Whitney U test (p > 0.05) (Table 3). Since describing the whole-organ VOI was impractical in a clinical workflow, quantitative studies of 18F-FGln to determine such organ uptake values may be conducted instead of using the spherical/elliptical VOI method.
The difference in 18F-FGln biodistribution between healthy volunteers and oncological patients
The maintenance of bodily glutamine homeostasis requires a balance between the rates of net glutamine uptake and release by the various organs of the body, such as the liver, kidney, lung, intestine, and muscle [19, 20]. We compared the 18F-FGln accumulation in 11 major organs/tissues (brain, heart, lungs, liver, spleen, pancreas, kidneys, intestine, bone, red marrow, and muscle) between healthy subjects and cancer patients. Compared with healthy subjects, cancer patients had lower 18F-FGln activity (SUVmean) in most organs, especially the lungs, spleen, heart, and muscles, with statistically significant differences (p < 0.05) (Table 4).
Best imaging phase and characteristics of the time-activity curves obtained from dynamic 18F-(2S,4R)4-fluoroglutamine PET scans in several cancer types
To define the variations in the radiopharmaceutical uptake among different regions of interest, the SUV values were helpful in assessing the PET data and estimating glutamine hypermetabolism. However, a critical issue was the time interval between the 18F-FGln injection and the measurements. To the best of our knowledge, there has been no systematic evaluation of the best imaging phase for 18F-FGln/PET in different cancer types. Therefore, we prospectively acquired imaging data at 10 min, 30 min, and 60 min postinjection in 31 cancer patients (17 breast cancer patients, 8 lung cancer patients, and 6 thyroid cancer patients).
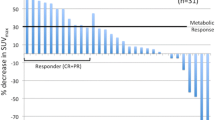
Upon visual inspection of the 18F-FGln PET/CT images, all three phases of the 18F-FGln PET/CT images indicated positive uptake in 29 of the 31 pathologically confirmed malignant primary tumors. However, other than for those breast cancers, the images acquired at the late phase (60 min) had greater visualization (contrast) of the positive 18F-FGln uptake in most of the primary tumors than those acquired in the early phase (10 min) (Fig. 4).
Quantitatively, in this dynamic study, we found that the peak of the SUVmax in breast and thyroid cancer tumors were within 10 min, while in lung cancer, the plateau of SUVmax was within 30 min to 60 min (Fig. 5), and there was no statistical difference between 30 min and 60 min, although SUVmax at 60 min showed a slight decrease (4.98 ± 0.16 vs. 4.75 ± 0.47, p > 0.05). The SURs of lung cancer (SUR10min = 3.61 ± 1.08; SUR30min = 5.29 ± 2.17; SUR60min = 7.13 ± 2.81) and thyroid cancer (SUR10min = 2.40 ± 1.97; SUR30min = 2.03 ± 1.55; SUR60min = 3.44 ± 2.40) showed an increase from the early-phase to the late-phase acquisition, and the p values were 0.046 and 0.794, respectively; however, a significant decrease was observed in the breast lesions between phases (SUR10min = 7.86 ± 2.16; SUR30min = 4.74 ± 1.88; SUR60min = 4.54 ± 2.24) (p = 0.022) (Supplemental Table S5). In parallel, the 18F-FGln uptake in the surrounding normal tissue of the lung and thyroid tumors consistently decreased over time, and the SUVmax values at 10 min postinjection were 1.69 ± 0.25 and 3.93 ± 0.15, which were then reduced to 0.92 ± 0.17 and 1.26 ± 0.58 at 60 min, respectively; this decrease of SUVmax in surrounding tissue at the late phase resulted in improved SUR values and visual effect of 18F-FGln metabolism in lung and thyroid tumors. Although a significant decrease in SUVmax (p = 0.008) and SUR (p = 0.022) was observed in breast cancers, the SUVmax values of most breast cancer lesions on the late-phase PET images were sufficient to meet the “avidity” definition of an uptake value greater than the blood pool activity [2]. The SUVmax and SUR values of 18F-FGln in different cancer patients at 10 min, 30 min, and 60 min scan were shown in Supplemental Table S5.
Tumor 18F-FGln avidity versus 18F-FDG avidity in patients with paired PET scans
With regard to the 18F-FGln dynamic PET/CT imaging in 17 breast cancer patients, two breast tumors, ductal carcinoma in situ, and mucinous carcinoma showed slight 18F-FGln activity (SUVmax < 3) at all stages (Supplemental Table S1). Nevertheless, two tumors appeared unclear on the 18F-FDG scan but were clear on 18F-FGln images (Fig. 6). Another 13 tumors and 6 metastatic lymph nodes were definitively diagnosed with the 18F-FGln image even though the SUVmax values were lower than the corresponding values on the 18F-FDG images (FGln-SUVmax = 4.61 ± 1.57, FDG-SUVmax = 9.25 ± 9.73, p < 0.05); the median SUVmax values for 18F-FGln and 18F-FDG were 4.54 and 6.4, respectively (Fig. 7a). In addition, as the Ki-67 proliferation index was currently used as a key marker of prognosis and since treatment guidelines are largely based on this index, we also investigated the correlation among the Ki-67 index, 18F-FGln uptake, and 18F-FDG uptake. Immunohistochemical staining confirmed that the SUVmax of 18F-FDG was positively correlated with the Ki-67 index (r = 0.75, p = 0.01), but the 18F-FGln SUVmax was not correlated with the Ki-67 index (r = − 0.09, p = 0.71) (Fig. 7b).
Additionally, 18F-FGln/PET could depict all 8 primary lung tumors. Both tracers showed similar increases in the radioactive uptake values of lung cancer patients, except for one patient who had bilateral symmetrical FDG avidity in the enlarged mediastinal lymph nodes with no detectable FGln avidity (Fig. 8). Finally, endobronchial ultrasound (EBUS)-guided flexible bronchoscopy and transbronchial needle aspiration (TBNA) confirmed that these positive subcarinal lymph nodes were epithelial tissue with carbon deposition and no tumor cells. All of these lesions were confirmed to have nonspecific FDG uptake during clinical follow-up and imaging with other modalities. Quantitatively, the SUVmax of all lung tumors during 1 h after the 18F-FGln injection ranged from 4.02 to 6.23 (lung cancer); all primary lung tumors could be clearly delineated by whole-body 18F-FGln and 18F-FDG PET/CT imaging, although 18F-FGln/PET did show a relatively lower SUVmax than 18F-FDG/PET (4.55 ± 1.00 vs. 7.82 ± 3.05, p < 0.05).
a18F-FDG and b18F-FGln PET/CT imaging in a 67-year-old woman with lung adenocarcinoma. Both tracers showed a 3.4-cm-sized tumor (red arrow) with spiculated margin in left inferior lobe (FDG-SUVmax = 8.01, FGln-SUVmax = 3.80). Some lymph nodes in subcarinal region and left hilum showed FDG-avid with no detectable FGln avidity (white arrow). These nodes were interpreted as benign according to histological analysis and clinical follow-up and imaging with other modalities
Furthermore, in 5 of the 6 primary thyroid tumors, similar differences in radiopharmaceutical avidity were observed, and the SUVmax values were 4.47 ± 0.50 (FGln) and 5.32 ± 2.08 (FDG) (p < 0.05). However, some small metastatic lymph nodes in thyroid cancer patients might show low 18F-FDG and 18F-FGln uptake during a 1-h scan and thus be missed because of the limited spatial resolution of PET imaging.
Discussion
Glutamine is the most abundant amino acid in the human body and is a constant source of metabolic energy as well as a source of carbon and nitrogen building blocks for new and rapidly dividing cells [21, 22]. Acute dependence on glutamine for growth is particularly evident in various cancer cells, many of which display oncogene-dependent addictions to glutamine in cell culture [23, 24]. Abnormal glutamine metabolism may be useful as a potential tumor biomarker, and 18F-FGln imaging has been evaluated in several different cancer types [2]. Studying glutamine metabolism by PET/CT may provide information about tumor location and the status of its energy production. To further investigate the biodistribution and tumor uptake and retention of 18F-FGln, the results of a series of dynamic PET/CT imaging studies for both healthy subjects and oncological patients after an IV injection of 18F-FGln were reported and compared.
18F-FGln displayed a biodistribution profile dominated by fast uptake and was excreted via the kidneys. The liver and gastrointestinal system also played major roles in uptake and retention [25]. Glutamine was utilized by the small intestine and renal epithelial cells for acid-base balance [26, 27]. In addition, 18F-FGln most likely had significant uptake in the pancreas because of its exocrine function and high rates of amino acid and protein turnover [7]. The organs with high levels of protein synthesis or cell proliferation, such as the salivary glands, thyroid, and stomach, showed moderate uptake. Additionally, the very low uptake in the brain, breast, lungs, and muscle could represent an advantage in PET imaging. In our study, 18F-FGln/PET demonstrated higher uptake in the trabecular bone of the ribs, vertebrae, and pelvis, which are rich in red marrow; this finding may be because the proliferation of rapidly dividing bone marrow-derived cells is strongly dependent on the availability of free glutamine, whose uptake might be mediated through different amino acid transporters [28]. The data from a study by Dass et al. also supported the fact that glutamine, but not glucose, is critically required in myelopoiesis [29].
The variations in the uptake of normal organs on the 18F-FGln PET scan, which were measured by the CV, need to be understood to allow the uptake changes in malignant lesions to be confidently attributed to changes in disease or treatment response rather than inherent variability in the scan. For these reasons, we addressed the quantitative aspects of 18F-FGln imaging as they relate to normal organ variability. In this study, we assessed the variability of 18F-FGln uptake in 20 major organs. The liver uptake showed a low variability (CV = 14.70%) when using SUVmean, which was similar to that of 18F-FDG/PET (13.35%). Because the liver has a moderate level of 18F-FDG uptake in the normal parenchyma and a low uptake variability relative to that of other organs, this organ was chosen as the basis of quantitative reliability in PET images [30]; our data imply that the liver could also be chosen as the basis of quantitative reliability in 18F-FGln PET images. Furthermore, the 18F-FGln CV of the breast was low, indicating that the detection of breast lesions in 18F-FGln images may be less disturbed by background tissue compared with 18F-FDG.
Because of the varied roles of glutamine in normal cell physiology and metabolism, the alterations in glutamine metabolism are expected to contribute to neoplastic transformation and cancer progression [19, 31]. Furthermore, a series of interrelated adaptive changes may occur in different organs in response to proliferating cancer cells using a disproportionate amount of glutamine [32]. Our study supports the recent findings that proliferating cancer cells compete with normal cells for circulating glutamine; therefore, low blood glutamine levels may induce reciprocal adaptive changes in the organs involved in body glutamine balance, such as the skeletal muscles, lungs, heart, and spleen [33]. The decreased SUVmean of muscle may be caused by an increase in the activity of the glutaminase enzyme and a consequent increase in the intracellular glutamine storage of muscle cells [34, 35]. Similarly, the lungs play a key role in glutamine production in cancer patients [20], and our results support this conclusion. In response to the diminished amount of circulating glutamine in the body, lung cells may preserve their glutamine transport activity and metabolism by inducing glutamine synthase expression as a consequence of glucocorticoid signaling and other mechanisms [36]. This may explain why the 18F-FGln activity (SUVmean) was lower in the lungs of cancer patients than in those of the healthy group. The metabolic changes induced by altered glutamine metabolism in the liver are more complicated than those in the lung. Hepatocytes serve both as a glutamine producer and consumer depending on the overall metabolic needs of the body [11]. This dual function may be the reason why the SUVmeans of the liver in healthy subjects and that in cancer patients were not significantly different. On the other hand, this result, along with its low uptake variability, further suggests that the liver should be chosen as the basis of quantitative reliability in 18F-FGln/PET images.
Furthermore, in this study we found that the SUVmax in most tumors could reach its maximum value within 60 min, so we prospectively evaluated a 1-h acquisition protocol in 31 cancer patients to obtain the highest SUR for visualizing tumors and quantifying tumor parameters. The SUVmax and SUR in the 18F-FGln PET images recorded at 10 min, 30 min, and 60 min postinjection were compared. These results suggest that late imaging (60 min) should be used for lung and thyroid cancer patients and conducting 18F-FGln PET/CT imaging 10 min after the tracer injection may facilitate the best contrast resolution (SUR) in breast cancer patients. However, the SUR of breast cancer lesions on the late-phase images, while significantly low, was still adequate for the diagnosis of tumors.
An in-depth investigation was performed with 17 breast cancer patients to fully evaluate the imaging characteristics of 18F-FGln/PET for breast cancer. The metabolic rate of glutamine in the normal breast tissue was relatively low. Breast density may also affect the apparent uptake of 18F-FGln. However, the ability to discriminate between benign and invasive lesions was unlikely to be affected by the breast density since the calculated average SUVs were low, and the peak SUV never exceeded 1.01. In clinical practice, the association between PET parameters such as SUVmax and the expression of the proliferation marker Ki-67 is very important. Ki-67 is a nonhistone nucleoprotein that can be synthesized throughout all phases of the cell cycle, except for the G0 phase [37]. In breast cancer, high Ki-67 expression is associated with a higher risk of death and recurrence than low expression of Ki-67 [38]. However, whether the SUVmax can reflect the proliferative activity of breast cancers and the correlation between PET parameters and Ki-67 expression are still controversial [14]. In this study, 18F-FDG showed a higher positive correlation between the SUVmax of breast cancer and the Ki-67 index (r = 0.75, p = 0.01) than 18F-FGln (r = − 0.09, p = 0.71). Additionally, the cellular and molecular mechanisms that drive 18F-FGln uptake in cancer patients are very difficult to study and remain poorly understood. Further investigations will be needed to clarify their relationships.
In our study, the FGln-avid tumors did not necessarily showed FDG-avid and vice versa, which contrasts with the results reported by Dunphy et al., who revealed a correlation between FGln avidity and FDG avidity approaching significance with Fisher’s exact test (p = 0.07) in 17 of the 25 patients (breast, pancreas, renal, neuroendocrine, lung, colon, lymphoma, bile duct, or glioma) [2]. This disagreement could be due to different patient populations and tumor biology characteristics. In addition, although the positive predictive value of 18F-FDG PET/CT was high, the specificity of this modality to differentiate benign lymph node involvement from malignant lymph node involvement was low [39]. In our study, 18F-FDG was not an ideal tracer for identifying benign and malignant mediastinal lymph nodes, while 18F-FGln appeared to be a suitable agent for these situations. In the future, additional cancer patients will need to be enrolled for 18F-FGln/PET imaging studies, and the results will provide sufficient statistical power to differentiate between the different types of tumor metabolism that drive proliferation. An increased understanding of tumor metabolism could be essential not only for assisting diagnoses but also for better patient management strategies based on their tumor metabolism status.
Conclusion
The images provided by 18F-FGln/PET may further supplement the diagnostic ability of 18F-FDG for cancer patients and detect metabolic changes in different tumors. Furthermore, late-phase imaging (60 min) could be chosen for most cancer patients; however, conducting 18F-FGln PET/CT 10 min after the tracer injection may facilitate the best contrast resolution (SUR) in breast cancer patients.
References
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3:169–80.
Dunphy MPS, Harding JJ, Venneti S, Zhang H, Burnazi EM, Bromberg J, et al. In vivo PET assay of tumor glutamine flux and metabolism: in-human trial of (18)F-(2S,4R)-4-fluoroglutamine. Radiology. 2018;287:667–75.
Zhu L, Ploessl K, Zhou R, Mankoff D, Kung HF. Metabolic imaging of glutamine in cancer. J Nucl Med. 2017;58:533–7.
Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7:274ra17.
Xu X, Zhu H, Liu F, Zhang Y, Yang J, Zhang L, et al. Imaging brain metastasis patients with 18F-(2S,4R)-4-fluoroglutamine. Clin Nucl Med. 2018;43:e392–e9.
Liu F, Xu X, Zhu H, Zhang Y, Yang J, Zhang L, et al. PET imaging of (18)F-(2 S,4 R)4-fluoroglutamine accumulation in breast cancer: from xenografts to patients. Mol Pharm. 2018;15:3448–55.
Lieberman BP, Ploessl K, Wang L, Qu W, Zha Z, Wise DR, et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med. 2011;52:1947–55.
Ploessl K, Wang L, Lieberman BP, Qu W, Kung HF. Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J Nucl Med. 2012;53:1616–24.
Qu W, Zha Z, Ploessl K, Lieberman BP, Zhu L, Wise DR, et al. Synthesis of optically pure 4-fluoro-glutamines as potential metabolic imaging agents for tumors. J Am Chem Soc. 2011;133:1122–33.
Zhou R, Pantel AR, Li S, Lieberman BP, Ploessl K, Choi H, et al. [(18)F](2S,4R)4-fluoroglutamine PET detects glutamine pool size changes in triple-negative breast cancer in response to glutaminase inhibition. Cancer Res. 2017;77:1476–84.
Zhu H, Liu F, Zhang Y, Yang J, Xu X, Guo X, et al. (2S,4R)-4-[(18)F] Fluoroglutamine as a PET indicator for bone marrow metabolism dysfunctional: from animal experiments to clinical application. Mol Imaging Biol. 2019.
Li X, Rowe SP, Leal JP, Gorin MA, Allaf ME, Ross AE, et al. Semiquantitative parameters in PSMA-targeted PET imaging with (18)F-DCFPyL: variability in normal-organ uptake. J Nucl Med. 2017;58:942–6.
Morioka T, Niikura N, Kumaki N, Masuda S, Iwamoto T, Yokoyama K, et al. Comparison of Ki-67 labeling index measurements using digital image analysis and scoring by pathologists. Breast Cancer. 2018;25:768–77.
Niikura N, Masuda S, Kumaki N, Xiaoyan T, Terada M, Terao M, et al. Prognostic significance of the Ki67 scoring categories in breast cancer subgroups. Clin Breast Cancer. 2014;14:323–9.e3.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Kessler RM, Seibyl J, Cowan RL, Zald D, Young JS, Ansari MS, et al. Radiation dosimetry of (18)F-FPEB in humans. J Nucl Med. 2014;55:1119–21.
Hoglund J, Shirvan A, Antoni G, Gustavsson SA, Langstrom B, Ringheim A, et al. 18F-ML-10, a PET tracer for apoptosis: first human study. J Nucl Med. 2011;52:720–5.
FDA. Tetle 21, Code of Federal Regulations, Part 361: Radioactive Drugs for Certain Research Uses; 1985.
Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–84.
Souba WW, Herskowitz K, Plumley DA. Lung glutamine metabolism. JPEN J Parenter Enteral Nutr. 1990;14:68S–70S.
Rajagopalan KN, DeBerardinis RJ. Role of glutamine in cancer: therapeutic and imaging implications. J Nucl Med. 2011;52:1005–8.
Shanware NP, Mullen AR, DeBerardinis RJ, Abraham RT. Glutamine: pleiotropic roles in tumor growth and stress resistance. J Mol Med (Berl). 2011;89:229–36.
Pantel AR, Ackerman D, Lee SC, Mankoff DA, Gade TP. Imaging cancer metabolism: underlying biology and emerging strategies. J Nucl Med. 2018;59:1340–9.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47.
Neis EP, Sabrkhany S, Hundscheid I, Schellekens D, Lenaerts K, Olde Damink SW, et al. Human splanchnic amino-acid metabolism. Amino Acids. 2017;49:161–72.
Brosnan JT. Interorgan amino acid transport and its regulation. J Nutr. 2003;133:2068S–72S.
Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36:1302–15.
Raposo B, Vaartjes D, Ahlqvist E, Nandakumar KS, Holmdahl R. System A amino acid transporters regulate glutamine uptake and attenuate antibody-mediated arthritis. Immunology. 2015;146:607–17.
Dass PD, Murdoch FE, Wu MC. Glutamine promotes colony formation in bone marrow and HL-60 cells; accelerates myeloid differentiation in induced HL-60 cells. In Vitro. 1984;20:869–75.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31.
Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, et al. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153–63.
Mohamed A, Deng X, Khuri FR, Owonikoko TK. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 2014;15:7–15.
Luo Y, Yoneda J, Ohmori H, Sasaki T, Shimbo K, Eto S, et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014;74:330–40.
Chen Q, Kirk K, Shurubor YI, Zhao D, Arreguin AJ, Shahi I, et al. Rewiring of glutamine metabolism is a bioenergetic adaptation of human cells with mitochondrial DNA mutations. Cell Metab. 2018;27:1007–25 e5.
Patterson BW, Horowitz JF, Wu G, Watford M, Coppack SW, Klein S. Regional muscle and adipose tissue amino acid metabolism in lean and obese women. Am J Physiol Endocrinol Metab. 2002;282:E931–6.
Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153:477–91.
Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology. 2017;49:166–71.
Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23:1409–15.
Acknowledgements
This work was supported in part by grants from the Beijing Natural Science Foundation Key Program (no. 7171002), National Natural Science Foundation of China (81571705, 81671733, 81871386, and 81871387), Beijing Municipal Administration of Hospitals-Yangfan Project (ZYLX201816), Beijing Nova Program (Z171100001117020), and Beijing Excellent Talents Funding (2017000021223ZK33).
Funding
This study was funded by the Beijing Natural Science Foundation Key Program (no. 7171002), National Natural Science Foundation of China (81571705, 81671733, 81871386, and 81871387), Beijing Municipal Administration of Hospitals-Yangfan Project (ZYLX201816), Beijing Nova Program (Z171100001117020), and Beijing Excellent Talents Funding (2017000021223ZK33).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – General
Electronic supplementary material
ESM 1
(DOCX 781 kb)
Rights and permissions
About this article
Cite this article
Xu, X., Zhu, H., Liu, F. et al. Dynamic PET/CT imaging of 18F-(2S, 4R)4-fluoroglutamine in healthy volunteers and oncological patients. Eur J Nucl Med Mol Imaging 47, 2280–2292 (2020). https://doi.org/10.1007/s00259-019-04543-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04543-w