Abstract
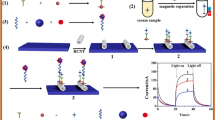
In this work, an electrochemiluminescence (ECL) biosensor was fabricated for the selective detection of vascular endothelial growth factor (VEGF165). g-C3N4/PDDA/CdSe nanocomposites were used as the ECL substrate. Then, DNA labeled at the 5′ end with amino groups (DNA1) was immobilized on the surface of g-C3N4/PDDA/CdSe nanocomposite-modified glassy carbon electrode (GCE) by amido linkage. AuNP-labeled target DNA (Au-DNA2) could hybridize with DNA1 to form a double strand. The ECL of the g-C3N4/PDDA/CdSe nanocomposite was efficiently quenched due to the resonance energy transfer between CdSe QDs and Au NPs. After VEGF165 was recognized and bound by Au-DNA2, the double helix was disrupted, and the energy transfer was broken. In this case, Au-DNA2 was released from the electrode surface, and the ECL intensity recovered to a higher level. Under optimal conditions, this ECL biosensor possesses excellent selectivity, accuracy, and stability for VEGF165 detection in a linear range of 2 pg mL−1 to 2 ng mL−1 with a detection limit of 0.68 pg mL−1. In addition, this assay has been successfully applied to the determination of VEGF165 in serum samples.

Schematic representation of the electrochemiluminescence sensor based on a g-C3N4/PDDA/CdSe nanocomposite, which can be determined in the concentration of vascular endothelial growth factor in serum.





Similar content being viewed by others
References
Senger DR, Galli SJ, Dvorak AM, Perruzzi C, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Sci. 1983;21:9983–5.
Keck PJ, Hauser SD, Krivi GG, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Sci. 1989;246:9–12.
Ahmetov II, Williams AG, Popov DV, Lyubaeva EV, Hakimullina AM, Fedotovskaya ON, et al. Hum Genet. 2009;12:126–751.
Aiello LP, Pierce EA, Foley E, Takagi H, Chen HH, Riddle L, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. P Natl Acad Sci USA. 1995;92:10457–61.
Omar AR. Targeting vascular endothelial growth factor (VEGF) pathway in gastric cancer: preclinical and clinical aspects. Crit Rev Oncol Hematol. 2015;93:18–27.
Do DV, Nguyen QD, Vitti R, Berliner AJ, Gibson A, Saroj N, et al. Intravitreal aflibercept injection in diabetic macular edema patients with and without prior anti-vascular endothelial growth factor treatment. OpHth. 2016;123:850–7.
Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti–interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;8:1521–9.
Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nat. 1992;359:845–8.
Slawomir L, Grazyna E B, Ewa G S, Maciej S, The plasma concentration of VEGF, HE4 and CA125 as a new biomarkers panel in different stages and sub-types of epithelial ovarian tumors.
Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. ACCR. 1995;5:487–91.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nat. 2000;407:249–57.
Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol. 2004;122.
Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. BioEssays. 2004;26:943–54.
Tsai M, Wang T, Lin Y, Kuo P, Su YH, Huang H. Aryl hydrocarbon receptor agonists upregulate VEGF secretion from bronchial epithelial cells. J Mol Med. 2015;93:1257–69.
Li JL, Sun KX, Chen ZP, Shi JF, Zhou DD, Xie GM. A fluorescence biosensor for VEGF detection based on DNA assembly structure switching and isothermal amplification. Biosens Bioelectron. 2017;89:964–9.
Iliuk A, Hu LH, Tao A. Aptamer in bioanalytical applications. Anal Chem. 2011;83:4440–52.
Liu HY, Xu SM, He ZM, Deng AP, Zhu JJ. Supersandwich cytosensor for selective and ultrasensitive detection of cancer cells using aptamer-DNA concatamer-quantum dots probes. Anal Chem. 2013;85:3385–92.
Peng L, Wu CCS, You MX, Han D, Chen Y, Fu T, et al. Engineering and applications of DNA-grafted polymer materials. Chem Sci. 2013;4:1928–38.
Yang XJ, Liu X, Liu Z, Pu F, Ren JS, Qu XG. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv Mater. 2012;24:2890–5.
Zhao J, He XL, Bo B, Liu XJ, Yin YM, Li GX. A signal-on electrochemical aptasensor for simultaneous detection of two tumor markers. Biosens Bioelectron. 2012;34:249–52.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Sci. 1990;249:505–10.
Cho EJ, Lee JW, Ellington AD. Applications of aptamers as sensors. Rev Anal Chem. 2009;2:241–64.
Hianik T, Wang J. Electrochemical aptasensors - recent achievements and perspectives. Electroanaly. 2009;21:1223–35.
Song SP, Wang LH, Li J, Zhao JL, Fan CH. Aptamer-based biosensors. Anal Chem. 2008;27:108–17.
Suzuki Y, Yokoyama K. Development of a fluorescent peptide for the detection of vascular endothelial growth factor (VEGF). Bioconjug Chem. 2009;10:1793–5.
Scapini P, Calzetti F, Cassatella MA. On the detection of neutrophil-derived vascular endothelial growth factor VEGF. J Immunol Methods. 1999;232:121–9.
Zhou H, Yue H, Zhou YL, Wang L, Fu ZF. A novel disposable immunosensor based on quenching of electrochemiluminescence emission of Ru(bpy)32+ by amorphous carbon nanoparticles. Sensors Actuators B Chem. 2015;209:744–50.
Chen Z, Ma G, Chen ZH, Zhang YG, Zhang Z, Gao JW, et al. Fabrication and photoelectrochemical properties of silicon nanowires/g-C3N4 Core/shell arrays. Appl Surf Sci. 2017;396:609–15.
Li RY, Zhang Y, Tu WW, Dai ZH. A photoelectrochemical bioanalysis platform for cells monitoring based on dual signal amplification using in situ generation of electron acceptor coupled with heterojunction. ACS. 2017;9:22289–97.
Wang HY, Zhang QH, Yin HS, Wang MH, Jiang WJ, Ai SY. A photoelectrochemical immunosensor for methylated RNA detection based on g-C3N4/CdS quantum dots heterojunction and Phos-tag-biotin. Biosens Bioelectron. 2017;95:124–30.
Zhang H, Li MX, Li CH, Guo ZH, Dong HL, Wu P, et al. G-Quadruplex DNAzyme-based electrochemiluminescence biosensing strategy for VEGF165 detection: combination of aptamer–target recognition and T7 exonuclease-assisted cycling signal amplification. Biosens Bioelectron. 2015;74:98–103.
Pang XH, Bian HJ, Wang WJ, Liu C, Khan MS, Wang Q, et al. A bio-chemical application of N-GQDs and g-C3N4 QDs sensitized TiO2 nanopillars for the quantitative detection of pcDNA3-HBV. Biosens Bioelectron. 2017;91:456–64.
Zhao M, Fan GC, Chen JJ, Shi JJ, Zhu JJ. Highly sensitive and selective photoelectrochemical biosensor for Hg2+ detection based on dual signal amplification by exciton energy transfer coupled with sensitization effect. Anal Chem. 2015;87:12340–7.
Zhang XR, Li SG, Jin X, Li XM. Aptamer based photoelectrochemical cytosensor with layer-by-layer assembly of CdSe semiconductor nanoparticles as photoelectrochemically active species. Biosens Bioelectron. 2011;26:3674–8.
Jie GF, Chen K, Wang XC, Lu ZK. Dual-stabilizer-capped CdSe quantum dots for off-on electrochemiluminescence biosensing of thrombin by target-triggered multiple amplification. RSC Adv. 2016;6:2065–71.
Du XL, Kang TF, Lu LP, Cheng SY. An electrochemiluminescence sensor based on CdSe@CdS functionalized MoS2 and hemin/Gquadruplex-based DNAzyme biocatalytic precipitation for sensitive detection of Pb(II). Anal Methods. 2018:51–8.
Kopra K, Syrjanp M, Hanninen P, Harma H. Non-competitive aptamer-based quenching resonance energy transfer assay for homogeneous growth factor quantification. Analyst. 2014;139:2016–23.
bMita C, bAbeK, Fukaya T, Ikebukuro K. Vascular endothelial growth factor (VEGF) detection using an aptamer and PNA-based bound/free separation system, Materials. Mater. 2014;7:1046–54.
Wang SE, Huang Y, Hu K, Tian JN, Zhao SL. A highly sensitive and selective aptasensor based on fluorescence polarization for the rapid determination of oncoprotein vascular endothelial growth factor (VEGF). Anal Methods. 2014;6:62–6.
Nonaka Y, Abe K, Ikebukuro K. Electrochemical detection of vascular endothelial growth factor with aptamer sandwich. Electrochem. 2012;80:363–6.
Zhao J, He XL, Bo B, Liu XJ, Yin YM, Li GX. A “signal-on” electrochemical aptasensor for simultaneous detection of two tumor markers. Biosens Bioelectron. 2012;34:249–52.
Abe K, Hasegawa H, Ikebukuro K. Electrochemical detection of vascular endothelial growth factor by an aptamer-based bound/free separation system. Electrochem. 2012;80:348–52.
Du XL, Lu LP, Cheng SY. An electrochemiluminescence sensor based on CdSe@CdS functionalized MoS2 and hemin/Gquadruplex-based DNAzyme biocatalytic precipitation for sensitive detection of Pb(II). Anal Methods. 2018:10–51.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21575001, 21976001), the Natural Science Foundation of Anhui Province (1508085MB37), and the Open fund for Discipline Construction of Institute of Physical Science and Information Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Ethical approval
The protocol of this study was approved by the medical ethics committee of The First Affiliated Hospital of Anhui Medical University. The blood samples (2 mL) of the participants were collected after informed written consent was provided by the subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, Jl., Liu, XP., Chen, JS. et al. Highly sensitive electrochemiluminescence biosensor for VEGF165 detection based on a g-C3N4/PDDA/CdSe nanocomposite. Anal Bioanal Chem 412, 3073–3081 (2020). https://doi.org/10.1007/s00216-020-02552-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02552-5