Abstract
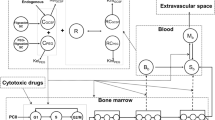
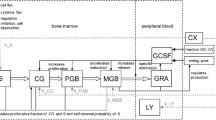
Neutropenia is one of the most common dose-limiting toxocities associated with anticancer drug therapy. The ability to predict the probability and severity of neutropenia based on in vitro studies of drugs in early drug development will aid in advancing safe and efficacious compounds to human testing. Toward this end, a physiological model of granulopoiesis and its regulation is presented that includes the bone marrow progenitor cell cycle, allowing for a mechanistic representation of the action of relevant anticancer drugs based on in vitro studies. Model development used data from previously reported tracer kinetic studies of granulocyte disposition in healthy humans to characterize the dynamics of neutrophil margination in the presence of endogenous granulocyte-colony stimulating factor (G-CSF). In addition, previously published data from healthy volunteers following pegfilgrastim and filgrastim were used to quantify the regulatory effects of support G-CSF therapies on granulopoiesis. The model was evaluated for the cell cycle inhibitor palbociclib, using an in vitro system of human bone marrow mononuclear cells to quantify the action of palbociclib on proliferating progenitor cells, including its inhibitory effect on G1 to S phase transition. The in vitro results were incorporated into the physiological model of granulopoiesis and used to predict the time course of absolute neutrophil count (ANC) and the incidence of neutropenia observed in three previously reported clinical trials of palbociclib. The model was able to predict grade 3 and 4 neutropenia due to palbociclib treatment with 86% accuracy based on in vitro data.










Similar content being viewed by others
References
Pujo-Menjouet L (2016) Blood cell dynamics: half of a century of modelling. Math Modell Nat Phenom 11:92–115
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20:4713–4721. https://doi.org/10.1200/JCO.2002.02.140
Craig M (2017) Towards quantitative systems pharmacology models of chemotherapy-induced neutropenia. Pharm Syst Pharmacol 6(5):293–304
Roskos LK, Lum P, Lockbaum P, Schwab G, Yang BB (2006) Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol 46:747–757. https://doi.org/10.1177/0091270006288731
Krzyzanski W, Wiczling P, Lowe P, Pigeolet E, Fink M, Berghout A, Balser S (2010) Population modeling of filgrastim PK-PD in healthy adults following intravenous and subcutaneous administrations. J Clin Pharmacol 50:101S–112S. https://doi.org/10.1177/0091270010376966
Quartino AL, Karlsson MO, Lindman H, Friberg LE (2014) Characterization of endogenous G-CSF and the inverse correlation to chemotherapy-induced neutropenia in patients with breast cancer using population modeling. Pharm Res 31:3390–3403. https://doi.org/10.1007/s11095-014-1429-9
Schirm S, Engel C, Loeffler M, Scholz M (2014) Modelling chemotherapy effects on granulopoiesis. BMC Syst Biol 8:138. https://doi.org/10.1186/s12918-014-0138-7
Craig M, Humphries AR, Mackey MC (2016) A mathematical model of granulopoiesis incorporating the negative feedback dynamics and kinetics of G-CSF/neutrophil binding and internalization. Bull Math Biol 78(12):2304–2357. https://doi.org/10.1007/s11538-016-0179-8
Panetta JC, Kirstein MN, Gajjar AJ, Nair G, Fouladi M, Stewart CF (2003) A mechanistic mathematical model of temozolomide myelosuppression in children with high-grade gliomas. Math Biosci 186:29–41. https://doi.org/10.1016/j.mbs.2003.07.002
Mangas-Sanjuan V, Buil-Bruna N, Garrido MJ, Soto E, Trocóniz IF (2015) Semimechanistic cell-cycle type-based pharmacokinetic/pharmacodynamic model of chemotherapy-induced neutropenic effects of diflomotecan under different dosing schedules. J Pharmacol Exp Ther 354(1):55–64. https://doi.org/10.1124/jpet.115.223776
Fornari C, O’Connor LO, Pin C, Smith A, Yates JWT, Amy Cheung SY, Jodrell DI, Mettetal JT, Collins TA (2019) Quantifying drug-induced bone marrow toxicity using a novel haematopoiesis systems pharmacology model. Pharm Syst Pharm. https://doi.org/10.1002/psp4.12459
Mauer AM, Athens JW, Ashenbrucker H, Cartwright GE, Wintrobe MM (1960) Leukokinetic studies. II. A method for labeling granulocytes in vitro with radioactive diisopropylfluorophosphate (DFP32). J Clin Investig 39(9):1481–1486. https://doi.org/10.1172/JCI104167
Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, Sacaan AI (2016) Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 22(8):2000–2008. https://doi.org/10.1158/1078-0432.CCR-15-1421
Sun W, O’Dwyer PJ, Finn RS, Ruiz-Garcia A, Shapiro GI, Schwartz GK, DeMichele A, Wang D (2017) Characterization of neutropenia in advanced cancer patients following palbociclib treatment using a population pharmacokinetic-pharmacodynamic modeling and simulation approach. J Clin Pharmacol 00:1–15. https://doi.org/10.1002/jcph.902
National Cancer Institute (2016) Common terminology criteria for adverse events, version 4.0. 2009
Seita J, Weissman IL (2010) Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2(6):640–653. https://doi.org/10.1002/wsbm.86.Hematopoietic
Cheung TH, Rando TA (2013) Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14(6):329–340
Dalton S (2015) Linking the cell cycle to cell fate decisions. Trends Cell Biol 25(10):592–600
Nguyen-Jackson HT, Zhang H, Watowich SS (2012) G-CSF receptor structure, function, and intracellular signal transduction. In: Twenty years of G-CSF. Springer, New York, pp 83–105
Chatta GS, Price TH, Allen RC, Dale DC (1994) Effects of in vivo recombinant methionyl human granulocyte colony-stimulating factor on the neutrophil response and peripheral blood colony-forming cells in healthy young and elderly adult volunteers. Blood 84(9):2923–2929
Price TH, Chatta GS, Dale DC (1996) Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 88(1):335–340
Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS (2010) STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood 116:2462–2471. https://doi.org/10.1182/blood-2009-12-259630
Mehta HM, Malandra M, Corey SJ (2015) G-CSF and GM-CSF in neutropenia. J Immunol 195(4):1341–1349. https://doi.org/10.4049/jimmunol.1500861
Nygren JM, Bryder D, Jacobsen SEW (2006) Prolonged cell cycle transit is a defining and developmentally conserved hemopoietic stem cell property. J Immunol 177(1):201–208. https://doi.org/10.4049/jimmunol.177.1.201
Morgan DO (2007) The cell cycle: principles of control. New Science Press, London
Skubitz KM (2013) Neutrophilic leukocytes. In: Wintrobe’s clinical hematology: Thirteenth Edition. Wolters Kluwer Health Adis, Philadelphia (ESP)
Deubelbeiss KA, Dancey JT, Harker LA, Finch CA (1975) Neutrophil kinetics in the dog. J Clin Investig 55(4):833–839. https://doi.org/10.1172/JCI107994
Dancey JT, Deubelbeiss KA, Harker LA, Finch CA (1976) Neutrophil kinetics in man. J Clin Investig 58(3):705–715. https://doi.org/10.1172/JCI108517
Tak T, Tesselaar K, Pillay J, Borghans JAM, Koenderman L (2013) Whatˈs your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol 94:595–601. https://doi.org/10.1189/jlb.1112571
Price TH, Lee MY, Dale DC, Finch CA (1979) Neutrophil kinetics in chronic neutropenia. Blood 54:581–594
Borregaard N (2010) Neutrophils, from marrow to microbes. Immunity 33(5):657–670
Fedosov DA, Gompper G (2014) White blood cell margination in microcirculation. Soft Matter 10(17):2961–2970. https://doi.org/10.1039/c3sm52860j
Athens JW, Raab SO, Haab OP, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM (1961) Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J Clin Investig 40(1):159–164. https://doi.org/10.1172/JCI104230
Craddock CG, Perry S, Lawrence JS (1960) The dynamics of leukopenia and leukocytosis. Ann Intern Med 52:281–294
Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM (1961) Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Investig 40(6):989–995. https://doi.org/10.1172/JCI104338
Nakagawa M, Terashima T, D’yachkova Y, Bondy GP, Hogg JC, Van Eeden SF (1998) Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation 98(21):2307–2313. https://doi.org/10.1161/01.CIR.98.21.2307
Borleffs JCC, Bosschaert M, Vrehen HM, Schneider MME, Van Strijp J, Small MK, Borkett KM (1998) Effect of escalating doses of recombinant human granulocyte colony-stimulating factor (filgrastim) on circulating neutrophils in healthy subjects. Clin Ther 20(4):722–736. https://doi.org/10.1016/S0149-2918(98)80135-5
Katoh M, Shirai T, Shikoshi K, Ishii M, Saito M, Kitagawa S (1992) Neutrophil kinetics shortly after initial administration of recombinant human granulocyte colony-stimulating factor: neutrophil alkaline phosphatase activitv as an endorrenous marker. Eur J Haematol 49:19–24
Ulich TR, del Castillo J, Souza L (1988) Kinetics and mechanisms of recombinant human granulocyte-colony stimulating factor-induced neutrophilia. Am J Pathol 133(3):630–638
Roskos LK (2012) The clinical pharmacology of filgrastim and pegfilgrastim. In: Twenty years of G-CSF. Springer, New York, pp 41–60
Watari K, Asano S, Shirafuji N, Kodo H, Ozawa K, Takaku F, Kamachi S (1989) Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood 73(1):117–122
Robinson CR (2002) Reduce, reuse, and recycle. Nat Biotechnol 20:879–880
Nicola NA, Metcalf D (1985) Binding of 125I-labeled granulocyte colony-stimulating factor to normal murine hemopoietic cells. J Cell Physiol 124(2):313–321. https://doi.org/10.1002/jcp.1041240222
Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G (1996) Filgrastim (r-metHuG-CSF): the first 10 years. Blood 88:1907–1929
Pastor ML, Laffont CM, Gladieff L, Schmitt A, Chatelut E, Concordet D (2013) Model-based approach to describe g-csf effects in carboplatin-treated cancer patients. Pharm Res 30(11):2795–2807. https://doi.org/10.1007/s11095-013-1099-z
Lord BI, Woolford LB, Molineux G (2001) Kinetics of neutrophil production in normal and neutropenic animals during the response to filgrastim (r-metHu G-CSF) or filgrastim SD/01 (PEG-r-metHu G-CSF). Clin Cancer Res 7(7):2085–2090
Yan X, Chen Y, Krzyzanski W (2012) Methods of solving rapid binding target-mediated drug disposition model for two drugs competing for the same receptor. J Pharmacokinet Pharmacodyn 39(5):543–560. https://doi.org/10.1007/s10928-012-9267-z
Zamboni WC (2003) Pharmacokinetics of pegfilgrastim. Pharmacotherapy 23(8 Pt 2):9S–14S. https://doi.org/10.1592/phco.23.9.9S.32888
Brekkan A, Lopez-Lazaro L, Yngman G, Plan EL, Acharya C, Hooker AC, Kankanwadi S, Karlsson MO (2018) A population pharmacokinetic-pharmacodynamic model of pegfilgrastim. AAPS J 20(5):91. https://doi.org/10.1208/s12248-018-0249-y
D’Argenio DZ, Schumitzky A, Wang X (2009) ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
Sun W, Wang DD (2014) A population pharmacokinetic (PK) analysis of palbociclib (PD-0332991) in patients (PTS) with advanced solid tumors. Ann Oncol 25:154
Abkowitz JL, Catlin SN, McCallie MT, Guttorp P (2002) Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood 100(7):2665–2667. https://doi.org/10.1182/blood-2002-03-0822
Yang BB, Lum PK, Hayashi MM, Roskos LK (2004) polyethylene glycol modification of filgrastim results in decreased renal clearance of the protein in rats. J Pharm Sci 93(5):1367–1373. https://doi.org/10.1002/jps.20024
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER (2010) Neutrophil kinetics in health and disease. Trends Immunol 31(8):318–324
Banker MJ, Clark TH, Williams JA (2003) Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J Pharm Sci 92:967–974. https://doi.org/10.1002/jps.10332
Yu Y, Loi C-M, Hoffman J, Wang D (2017) Physiologically based pharmacokinetic modeling of palbociclib. J Clin Pharmacol 57(2):173–184. https://doi.org/10.1002/jcph.792
Krzyzanski W, Harrold JM, Wu LS, Perez-Ruixo JJ (2016) A cell-level model of pharmacodynamics-mediated drug disposition. J Pharmacokinet Pharmacodyn 43(5):513–527. https://doi.org/10.1007/s10928-016-9491-z
Soto E, Staab A, Freiwald M, Munzert G, Fritsch H, Döge C, Trocóniz IF (2010) Prediction of neutropenia-related effects of a new combination therapy with the anticancer drugs BI 2536 (a Plk1 inhibitor) and pemetrexed. Clin Pharmacol Ther 88:660–667. https://doi.org/10.1038/clpt.2010.148
Vélez De Mendizábal N, Martínez-Forero I, Garrido MJ, Bandrés E, García-Foncillas J, Segura C, Trocóniz IF (2010) A semi-physiological-based pharmacokinetic/pharmacodynamic model to describe the effects of topotecan on B-lymphocyte lineage cells. Pharm Res 27:431–441. https://doi.org/10.1007/s11095-009-0025-x
Iadocicco K, Monteiro LHA, Chaui-Berlinck JG (2002) A theoretical model for estimating the margination constant of leukocytes. BMC Physiol. https://doi.org/10.1186/1472-6793-2-1
Acknowledgements
This work was supported by grants from National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering (NIH/NIBIB) P41-EB001978 and the Alfred E. Mann Institute at USC (DZD). The work was presented originally at the American Conference on Pharmacometrics in 2018, San Diego, CA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W. Chen and D.Z. D’Argenio declare no conflict of interests. B. Boras, T. Sung, Y. Yu, J. Zheng, D. Wang, W. Hu, and M.E. Spilker are employees of Pfizer, Inc. These authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, W., Boras, B., Sung, T. et al. A physiological model of granulopoiesis to predict clinical drug induced neutropenia from in vitro bone marrow studies: with application to a cell cycle inhibitor. J Pharmacokinet Pharmacodyn 47, 163–182 (2020). https://doi.org/10.1007/s10928-020-09680-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-020-09680-6