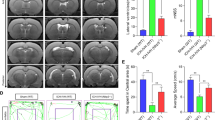
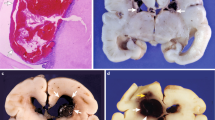
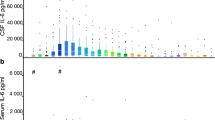
Abstract
Hydrocephalus is the most common neurosurgical disorder worldwide and is characterized by enlargement of the cerebrospinal fluid (CSF)-filled brain ventricles resulting from failed CSF homeostasis. Since the 1840s, physicians have observed inflammation in the brain and the CSF spaces in both posthaemorrhagic hydrocephalus (PHH) and postinfectious hydrocephalus (PIH). Reparative inflammation is an important protective response that eliminates foreign organisms, damaged cells and physical irritants; however, inappropriately triggered or sustained inflammation can respectively initiate or propagate disease. Recent data have begun to uncover the molecular mechanisms by which inflammation — driven by Toll-like receptor 4-regulated cytokines, immune cells and signalling pathways — contributes to the pathogenesis of hydrocephalus. We propose that therapeutic approaches that target inflammatory mediators in both PHH and PIH could address the multiple drivers of disease, including choroid plexus CSF hypersecretion, ependymal denudation, and damage and scarring of intraventricular and parenchymal (glia–lymphatic) CSF pathways. Here, we review the evidence for a prominent role of inflammation in the pathogenic mechanism of PHH and PIH and highlight promising targets for therapeutic intervention. Focusing research efforts on inflammation could shift our view of hydrocephalus from that of a lifelong neurosurgical disorder to that of a preventable neuroinflammatory condition.
Key points
-
Hydrocephalus, that is, the enlargement of brain ventricles associated with failed cerebrospinal fluid (CSF) homeostasis, is the most common neurosurgical disorder and is treated mainly by neurosurgical CSF diversion procedures with high rates of morbidity and failure.
-
Posthaemorrhagic hydrocephalus and postinfectious hydrocephalus are the most common causes of hydrocephalus and are both characterized by inflammation in the brain tissue and CSF space.
-
Recent data have begun to uncover the molecular mechanisms by which inflammation, driven by activation of Toll-like receptor 4, contributes to the pathogenesis of hydrocephalus.
-
Pharmacotherapeutic approaches that target inflammation have the potential to address multiple drivers of posthaemorrhagic hydrocephalus and postinfectious hydrocephalus, including acute hypersecretion of CSF by the choroid plexus epithelium and scarring of CSF drainage pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rekate, H. L. A contemporary definition and classification of hydrocephalus. Semin. Pediatr. Neurol. 16, 9–15 (2009).
Benveniste, H., Lee, H. & Volkow, N. D. The glymphatic pathway: waste removal from the CNS via cerebrospinal fluid transport. Neuroscientist 23, 454–465 (2017).
Brinker, T., Stopa, E., Morrison, J. & Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11, 10 (2014).
Furey, C. G. et al. De novo mutation in genes regulating neural stem cell fate in human congenital hydrocephalus. Neuron 99, 302–314.e4 (2018).
Kahle, K. T., Kulkarni, A. V., Limbrick, D. D. Jr. & Warf, B. C. Hydrocephalus in children. Lancet 387, 788–799 (2016).
Karimy, J. K. et al. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg. Focus 41, E10 (2016).
Karimy, J. K. et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 23, 997–1003 (2017).
Dewan, M. C. et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J. Neurosurg. 130, 1065–1079 (2018).
Cherian, S., Whitelaw, A., Thoresen, M. & Love, S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 14, 305–311 (2004).
Strahle, J. et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res. 3, 25–38 (2012).
Reddy, G. K., Bollam, P. & Caldito, G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 81, 404–410 (2014).
Isaacs, A. M. et al. Age-specific global epidemiology of hydrocephalus: systematic review, metanalysis and global birth surveillance. PLOS ONE 13, e0204926 (2018).
Warf, B. C., Campbell, J. W. & Riddle, E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv. Syst. 27, 1063–1071 (2011).
Chen, Q. et al. Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. J. Neurol. Sci. 375, 220–230 (2017).
Tsitouras, V. & Sgouros, S. Infantile posthemorrhagic hydrocephalus. Childs Nerv. Syst. 27, 1595–1608 (2011).
Murphy, B. P. et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch. Dis. Child. Fetal Neonatal Ed. 87, F37–F41 (2002).
Warf, B. C. & East African Neurosurgical Research Collaboration. Pediatric hydrocephalus in East Africa: prevalence, causes, treatments, and strategies for the future. World Neurosurg. 73, 296–300 (2010).
Bir, S. C. et al. Epidemiology of adult-onset hydrocephalus: institutional experience with 2001 patients. Neurosurg. Focus 41, E5 (2016).
Chahlavi, A., El-Babaa, S. K. & Luciano, M. G. Adult-onset hydrocephalus. Neurosurg. Clin. North. Am. 12, 753–760 (2001).
Cioca, A., Gheban, D., Perju-Dumbrava, D., Chiroban, O. & Mera, M. Sudden death from ruptured choroid plexus arteriovenous malformation. Am. J. Forensic Med. Pathol. 35, 100–102 (2014).
Muir, R. T., Wang, S. & Warf, B. C. Global surgery for pediatric hydrocephalus in the developing world: a review of the history, challenges, and future directions. Neurosurg. Focus 41, E11 (2016).
Aziz, I. A. Hydrocephalus in the Sudan. J. R. Coll. Surg. Edinb. 21, 222–224 (1976).
Kamat, A. S., Gretschel, A., Vlok, A. J. & Solomons, R. CSF protein concentration associated with ventriculoperitoneal shunt obstruction in tuberculous meningitis. Int. J. Tuberculosis Lung Dis. 22, 788–792 (2018).
Aranha, A., Choudhary, A., Bhaskar, S. & Gupta, L. N. A randomized study comparing endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the management of hydrocephalus due to tuberculous meningitis. Asian J. Neurosurg. 13, 1140–1147 (2018).
Rajshekhar, V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol. India 57, 368–374 (2009).
Li, K. et al. Clinical features, long-term clinical outcomes, and prognostic factors of tuberculous meningitis in West China: a multivariate analysis of 154 adults. Expert Rev. Anti Infect. Ther. 15, 629–635 (2017).
Lee, L. V. Neurotuberculosis among Filipino children: an 11 years experience at the Philippine children’s medical center. Brain Dev. 22, 469–474 (2000).
van der Linden, V. et al. Association of severe hydrocephalus with congenital Zika syndrome. JAMA Neurol. 76, 203–210 (2019).
Kulkarni, A. V. et al. Endoscopic treatment versus shunting for infant hydrocephalus in Uganda. N. Engl. J. Med. 377, 2456–2464 (2017).
Li, L. et al. Association of bacteria with hydrocephalus in Ugandan infants. J. Neurosurg. Pediatr. 7, 73–87 (2011).
Thigpen, M. C. et al. Bacterial meningitis in the United States, 1998-2007. N. Engl. J. Med. 364, 2016–2025 (2011).
Pyrgos, V., Seitz, A. E., Steiner, C. A., Prevots, D. R. & Williamson, P. R. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLOS ONE 8, e56269 (2013).
Liu, J. et al. Ventriculoperitoneal shunts in non-HIV cryptococcal meningitis. BMC Neurol. 18, 58 (2018).
Schiff, S. J., Ranjeva, S. L., Sauer, T. D. & Warf, B. C. Rainfall drives hydrocephalus in East Africa. J. Neurosurg. Pediatr. 10, 161–167 (2012).
Warf, B. C. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J. Neurosurg. 103, 475–481 (2005).
Warf, B. C. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J. Neurosurg. 102, 1–15 (2005).
Stagno, V., Navarrete, E. A., Mirone, G. & Esposito, F. Management of hydrocephalus around the world. World Neurosurg. 79 (Suppl. 2), S23.e17–20 (2013).
Kulkarni, A. V. First treatment in infants with hydrocephalus: the case for shunt. Neurosurgery 63 (Suppl. 1), 73–77 (2016).
Drake, J. M., Kulkarni, A. V. & Kestle, J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv. Syst. 25, 467–472 (2009).
Kulkarni, A. V. et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J. Pediatr. 155, 254–259.e1 (2009).
Limbrick, D. D. Jr., Baird, L. C., Klimo, P. Jr., Riva-Cambrin, J. & Flannery, A. M. Pediatric hydrocephalus: systematic review and evidence-based guidelines task force. Part 4: cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J. Neurosurg. Pediatr. 14, 30–34 (2014).
Pindrik, J., Jallo, G. I. & Ahn, E. S. Complications and subsequent removal of retained shunt hardware after endoscopic third ventriculostomy: case series. J. Neurosurg. Pediatr. 11, 722–726 (2013).
Kulkarni, A. V. et al. Outcomes of CSF shunting in children: comparison of hydrocephalus clinical research network cohort with historical controls: clinical article. J. Neurosurg. Pediatr. 12, 334–338 (2013).
Kulkarni, A. V. et al. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67, 588–593 (2010).
Baird, L. C. First treatment in infants with hydrocephalus: the case for endoscopic third ventriculostomy/choroid plexus cauterization. Neurosurgery 63, 78–82 (2016).
Kulkarni, A. V. et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective hydrocephalus clinical research network study. J. Neurosurg. Pediatr. 14, 224–229 (2014).
Marques, F. et al. The choroid plexus in health and in disease: dialogues into and out of the brain. Neurobiol. Dis. 107, 32–40 (2017).
Ghersi-Egea, J. F. et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 135, 337–361 (2018).
Lauer, A. N., Tenenbaum, T., Schroten, H. & Schwerk, C. The diverse cellular responses of the choroid plexus during infection of the central nervous system. Am. J. Physiol. Cell Physiol. 314, C152–C165 (2018).
Damkier, H. H., Brown, P. D. & Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 93, 1847–1892 (2013).
International PHVD Drug Trial Group. International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. Lancet 352, 433–440 (1998).
Whitelaw, A., Kennedy, C. R. & Brion, L. P. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst. Rev. 2, CD002270 (2001).
Libenson, M. H., Kaye, E. M., Rosman, N. P. & Gilmore, H. E. Acetazolamide and furosemide for posthemorrhagic hydrocephalus of the newborn. Pediatr. Neurol. 20, 185–191 (1999).
Teppema, L. J. & Dahan, A. Acetazolamide and breathing. Does a clinical dose alter peripheral and central CO2 sensitivity? Am. J. Respir. Crit. Care Med. 160, 1592–1597 (1999).
Erker, T. et al. The bumetanide prodrug BUM5, but not bumetanide, potentiates the antiseizure effect of phenobarbital in adult epileptic mice. Epilepsia 57, 698–705 (2016).
Tollner, K. et al. A novel prodrug-based strategy to increase effects of bumetanide in epilepsy. Ann. Neurol. 75, 550–562 (2014).
Seelig, A., Gottschlich, R. & Devant, R. M. A method to determine the ability of drugs to diffuse through the blood-brain barrier. Proc. Natl Acad. Sci. USA 91, 68–72 (1994).
Larroche, J.-C. Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biol. Neonate 20, 287–299 (1972).
Omar, A. T. II, Bagnas, M. A. C., Del Rosario-Blasco, K. A. R., Diestro, J. D. B. & Khu, K. J. O. Shunt surgery for neurocutaneous melanosis with hydrocephalus: case report and review of the literature. World Neurosurg. 120, 583–589.e3 (2018).
Whitelaw, A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin. Neonatol. 6, 135–146 (2001).
Lategan, B., Chodirker, B. N. & Del Bigio, M. R. Fetal hydrocephalus caused by cryptic intraventricular hemorrhage. Brain Pathol. 20, 391–398 (2010).
Hill, A., Shackelford, G. D. & Volpe, J. J. A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics 73, 19–21 (1984).
Milhorat, T. H., Hammock, M. K., Davis, D. A. & Fenstermacher, J. D. Choroid plexus papilloma. I. Proof of cerebrospinal fluid overproduction. Childs Brain 2, 273–289 (1976).
Gram, M. et al. Extracellular hemoglobin–mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J. Neuroinflammation 11, 200 (2014).
Gram, M. et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J. Neuroinflammation 10, 100 (2013).
Simard, P. F. et al. Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl. Stroke Res. 2, 227–231 (2011).
Barichello, T. et al. Pathophysiology of neonatal acute bacterial meningitis. J. Med. Microbiol. 62, 1781–1789 (2013).
Bateman, G. A. & Brown, K. M. The measurement of CSF flow through the aqueduct in normal and hydrocephalic children: from where does it come, to where does it go? Childs Nerv. Syst. 28, 55–63 (2012).
Oi, S. & Di Rocco, C. Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv. Syst. 22, 662–669 (2006).
Oreskovic, D., Rados, M. & Klarica, M. Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience 354, 69–87 (2017).
Miyajima, M. & Arai, H. Evaluation of the production and absorption of cerebrospinal fluid. Neurol. Med. Chir. 55, 647–656 (2015).
Lohrberg, M. & Wilting, J. The lymphatic vascular system of the mouse head. Cell Tissue Res. 366, 667–677 (2016).
Olstad, E. W. et al. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr. Biol. 29, 229–241.e6 (2019).
Gao, C. et al. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J. Cereb. Blood Flow Metab. 34, 1070–1075 (2014).
Polis, B., Polis, L. & Nowoslawska, E. Surgical treatment of post-inflammatory hydrocephalus. Analysis of 101 cases. Childs Nerv. Syst. 35, 237–243 (2019).
Raouf, A., Zidan, I. & Mohamed, E. Endoscopic third ventriculostomy for post-inflammatory hydrocephalus in pediatric patients: is it worth a try? Neurosurg. Rev. 38, 149–155; discussion 155 (2015).
[No authors listed]. Acute hydrocephalus, or water in the head, an inflammatory disease, and curable equally and by the same means with other diseases of inflammation. Br. Foreign Med. Rev. 11, 151–158 (1841).
Davis, D. D. Acute Hydrocephalus, or Water in the Head: an Inflammatory Disease, and Curable Equally by the Same Means with Other Diseases of Inflammation (Taylor & Walton, 1840).
[No authors listed]. Hydrocephalus reconsidered; and its relations to inflammation and irritation of the brain defined, with cases from hospital and private practice. Prov. Med. Surg. J. 15, 16–17 (1851).
Sharma, S. et al. Cytokines do play a role in pathogenesis of tuberculous meningitis: a prospective study from a tertiary care center in India. J. Neurol. Sci. 379, 131–136 (2017).
Chaudhry, S. R. et al. Elevated systemic IL-6 levels in patients with aneurysmal subarachnoid hemorrhage is an unspecific marker for post-SAH complications. Int. J. Mol. Sci. 18, E2580 (2017).
Kitazawa, K. & Tada, T. Elevation of transforming growth factor-β1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke 25, 1400–1404 (1994).
Whitelaw, A., Christie, S. & Pople, I. Transforming growth factor- β1: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr. Res. 46, 576–580 (1999).
Mlakar, J. et al. Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958 (2016).
Ulfig, N., Bohl, J., Neudorfer, F. & Rezaie, P. Brain macrophages and microglia in human fetal hydrocephalus. Brain Dev. 26, 307–315 (2004).
Thwaites, G. E. et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. 6, 230–236 (2007).
Schurkamper, M., Medele, R., Zausinger, S., Schmid-Elsaesser, R. & Steiger, H. J. Dexamethasone in the treatment of subarachnoid hemorrhage revisited: a comparative analysis of the effect of the total dose on complications and outcome. J. Clin. Neurosci. 11, 20–24 (2004).
Gutierrez-Murgas, Y. M., Skar, G., Ramirez, D., Beaver, M. & Snowden, J. N. IL-10 plays an important role in the control of inflammation but not in the bacterial burden in S. epidermidis CNS catheter infection. J. Neuroinflammation 13, 271 (2016).
Hausler, M. et al. Murine gammaherpesvirus-68 infection of mice: a new model for human cerebral Epstein-Barr virus infection. Ann. Neurol. 57, 600–603 (2005).
Zhu, W. et al. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLOS ONE 9, e97423 (2014).
Harada, T., Takamoto, M., Jin, D. H., Tada, T. & Sugane, K. Young C3H mice infected with Toxoplasma gondii are a novel experimental model of communicating hydrocephalus. Neurol. Res. 29, 615–621 (2007).
Guo, J. et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res. 1594, 115–124 (2015).
Sansing, L. H. et al. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann. Neurol. 70, 646–656 (2011).
Wang, Y. C. et al. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44, 2545–2552 (2013).
Lattke, M., Magnutzki, A., Walther, P., Wirth, T. & Baumann, B. Nuclear factor κB activation impairs ependymal ciliogenesis and links neuroinflammation to hydrocephalus formation. J. Neurosci. 32, 11511–11523 (2012).
Galbreath, E., Kim, S. J., Park, K., Brenner, M. & Messing, A. Overexpression of TGF-β1 in the central nervous system of transgenic mice results in hydrocephalus. J. Neuropathol. Exp. Neurol. 54, 339–349 (1995).
Tada, T., Kanaji, M. & Kobayashi, S. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-β1. J. Neuroimmunol. 50, 153–158 (1994).
Lindvall-Axelsson, M., Hedner, P. & Owman, C. Corticosteroid action on choroid plexus: reduction in Na+-K+-ATPase activity, choline transport capacity, and rate of CSF formation. Exp. Brain Res. 77, 605–610 (1989).
Weiss, M. H. & Nulsen, F. E. The effect of glucocorticoids on CSF flow in dogs. J. Neurosurg. 32, 452–458 (1970).
Sato, O., Hara, M., Asai, T., Tsugane, R. & Kageyama, N. The effect of dexamethasone phosphate on the production rate of cerebrospinal fluid in the spinal subarachnoid space of dogs. J. Neurosurg. 39, 480–484 (1973).
Steffensen, A. B. et al. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 9, 2167 (2018).
Keep, R. F. & Jones, H. C. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res. Dev. Brain Res. 56, 47–53 (1990).
Praetorius, J. Water and solute secretion by the choroid plexus. Pflug. Arch. 454, 1–18 (2007).
Praetorius, J. & Damkier, H. H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol. 312, C673–C686 (2017).
Medzhitov, R. TLR-mediated innate immune recognition. Semin. Immunol. 19, 1–2 (2007).
Coorens, M. et al. Cathelicidins inhibit Escherichia coli-induced TLR2 and TLR4 activation in a viability-dependent manner. J. Immunol. 199, 1418–1428 (2017).
Skipor, J., Szczepkowska, A., Kowalewska, M., Herman, A. P. & Lisiewski, P. Profile of toll-like receptor mRNA expression in the choroid plexus in adult ewes. Acta Vet. Hung. 63, 69–78 (2015).
Rivest, S. Molecular insights on the cerebral innate immune system. Brain Behav. Immun. 17, 13–19 (2003).
Miyake, K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 19, 3–10 (2007).
Fang, H. et al. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 286, 30393–30400 (2011).
Tsan, M. F. & Gao, B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 76, 514–519 (2004).
Chen, S., Luo, J., Reis, C., Manaenko, A. & Zhang, J. Hydrocephalus after subarachnoid hemorrhage: pathophysiology, diagnosis, and treatment. Biomed. Res. Int. 2017, 8584753 (2017).
Okamoto, T. et al. Matrix metalloproteinases in infants with posthemorrhagic hydrocephalus. Early Hum. Dev. 84, 137–139 (2008).
Ehrchen, J. M., Sunderkotter, C., Foell, D., Vogl, T. & Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 86, 557–566 (2009).
Yang, B., Zhou, Z., Li, X. & Niu, J. The effect of lysophosphatidic acid on Toll-like receptor 4 expression and the nuclear factor-κB signaling pathway in THP-1 cells. Mol. Cell Biochem. 422, 41–49 (2016).
Kwon, M. S. et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int. J. Mol. Sci. 16, 5028–5046 (2015).
Demeestere, D., Libert, C. & Vandenbroucke, R. E. Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov. Today 20, 928–941 (2015).
Kleine, T. O. & Benes, L. Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A 69, 147–151 (2006).
Wang, Y. C. et al. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann. Neurol. 75, 876–889 (2014).
Cox, K. H., Cox, M. E., Woo-Rasberry, V. & Hasty, D. L. Pathways involved in the synergistic activation of macrophages by lipoteichoic acid and hemoglobin. PLOS ONE 7, e47333 (2012).
Berkes, J., Viswanathan, V. K., Savkovic, S. D. & Hecht, G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52, 439–451 (2003).
Wilson, R. et al. Upper respiratory tract viral infection and mucociliary clearance. Eur. J. Respir. Dis. 70, 272–279 (1987).
Doyle, W. J. et al. Nasal and otologic effects of experimental influenza A virus infection. Ann. Otol. Rhinol. Laryngol. 103, 59–69 (1994).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Nowarski, R., Jackson, R. & Flavell, R. A. The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell 168, 362–375 (2017).
Karimy, J. K. et al. A novel method to study cerebrospinal fluid dynamics in rats. J. Neurosci. Methods 241, 78–84 (2015).
Piechotta, K., Garbarini, N., England, R. & Delpire, E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl– cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J. Biol. Chem. 278, 52848–52856 (2003).
Shekarabi, M. et al. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 25, 285–299 (2017).
Yan, Y. & Merlin, D. Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation. World J. Gastroenterol. 14, 6115–6121 (2008).
Yan, Y. et al. Nuclear factor-κB is a critical mediator of Ste20-like proline-/alanine-rich kinase regulation in intestinal inflammation. Am. J. Pathol. 173, 1013–1028 (2008).
Thiagarajah, J. R., Donowitz, M. & Verkman, A. S. Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 12, 446–457 (2015).
Lin, T. J. et al. SPAK plays a pathogenic role in IgA nephropathy through the activation of NF-κB/MAPKs signaling pathway. Free Radic. Biol. Med. 99, 214–224 (2016).
Lan, C. C. et al. Inhibition of Na-K-Cl cotransporter isoform 1 reduces lung injury induced by ischemia-reperfusion. J. Thorac. Cardiovasc. Surg. 153, 206–215 (2017).
Yan, Y., Nguyen, H., Dalmasso, G., Sitaraman, S. V. & Merlin, D. Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform. Biochim. Biophys. Acta 1769, 106–116 (2007).
Polek, T. C., Talpaz, M. & Spivak-Kroizman, T. The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem. Biophys. Res. Commun. 343, 125–134 (2006).
Alessi, D. R. et al. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci. Signal. 7, re3 (2014).
Thastrup, J. O. et al. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem. J. 441, 325–337 (2012).
de Los, H. P. et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl– co-transporters. Biochem. J. 458, 559–573 (2014).
Lee, D., Lee, S. A., Shin, D. M. & Hong, J. H. Chloride influx of anion exchanger 2 was modulated by calcium-dependent spinophilin in submandibular glands. Front. Physiol. 9, 889 (2018).
Li, Q. et al. Targeting germinal matrix hemorrhage-induced overexpression of sodium-coupled bicarbonate exchanger reduces posthemorrhagic hydrocephalus formation in neonatal rats. J. Am. Heart Assoc. 7, e007192 (2018).
Kim, K. S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 6, 625–634 (2008).
Koedel, U., Klein, M. & Pfister, H.-W. New understandings on the pathophysiology of bacterial meningitis. Curr. Opin. Infect. Dis. 23, 217–223 (2010).
Deopujari, C. E., Padayachy, L., Azmi, A., Figaji, A. & Samantray, S. K. Neuroendoscopy for post-infective hydrocephalus in children. Childs Nerv. Syst. 34, 1905–1914 (2018).
Sellner, J., Tauber, M. G. & Leib, S. L. Pathogenesis and pathophysiology of bacterial CNS infections. Handb. Clin. Neurol. 96, 1–16 (2010).
Malley, R. et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl Acad. Sci. USA 100, 1966–1971 (2003).
Lahrtz, F., Piali, L., Spanaus, K. S., Seebach, J. & Fontana, A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J. Neuroimmunol. 85, 33–43 (1998).
Krebs, V. L., Okay, T. S., Okay, Y. & Vaz, F. A. Tumor necrosis factor-alpha, interleukin-1beta and interleukin-6 in the cerebrospinal fluid of newborn with meningitis. Arq. Neuropsiquiatr. 63, 7–13 (2005).
van Furth, A. M., Roord, J. J. & van Furth, R. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect. Immun. 64, 4883–4890 (1996).
Grandgirard, D. & Leib, S. L. Meningitis in neonates: bench to bedside. Clin. Perinatol. 37, 655–676 (2010).
Dessing, M. C. et al. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J. Infect. Dis. 197, 245–252 (2008).
Flo, T. H. et al. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164, 2064–2069 (2000).
Janot, L. et al. CD14 works with toll-like receptor 2 to contribute to recognition and control of Listeria monocytogenes infection. J. Infect. Dis. 198, 115–124 (2008).
Seki, E. et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169, 3863–3868 (2002).
Mook-Kanamori, B. B., Geldhoff, M., van der Poll, T. & van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 24, 557–591 (2011).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Mottahedin, A., Joakim, Ek,C., Truve, K., Hagberg, H. & Mallard, C. Choroid plexus transcriptome and ultrastructure analysis reveals a TLR2-specific chemotaxis signature and cytoskeleton remodeling in leukocyte trafficking. Brain Behav. Immun. 79, 216–227 (2019).
Jimenez, A. J., Dominguez-Pinos, M. D., Guerra, M. M., Fernandez-Llebrez, P. & Perez-Figares, J. M. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2, e28426 (2014).
Guerra, M. M. et al. Cell junction pathology of neural stem cells is associated with ventricular zone disruption, hydrocephalus, and abnormal neurogenesis. J. Neuropathol. Exp. Neurol. 74, 653–671 (2015).
Yung, Y. C. et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl Med. 3, 99ra87 (2011).
Bayatti, N. et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex 18, 1536–1548 (2008).
Rodriguez, E. M. et al. A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biol. Res. 45, 231–242 (2012).
McAllister, J. P. et al. Ventricular zone disruption in human neonates with intraventricular hemorrhage. J. Neuropathol. Exp. Neurol. 76, 358–375 (2017).
Castaneyra-Ruiz, L. et al. Blood exposure causes ventricular zone disruption and glial activation in vitro. J. Neuropathol. Exp. Neurol. 77, 803–813 (2018).
Liu, S. F., Ye, X. & Malik, A. B. Inhibition of NF-κB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 100, 1330–1337 (1999).
Hu, Y. et al. Melatonin protects against blood-brain barrier damage by inhibiting the TLR4/ NF-κB signaling pathway after LPS treatment in neonatal rats. Oncotarget 8, 31638–31654 (2017).
Robinson, S. et al. Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Front. Cell Neurosci. 12, 322 (2018).
Rice, T. W. et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 38, 1685–1694 (2010).
Allette, Y. M. et al. Decoy peptide targeted to Toll-IL-1R domain inhibits LPS and TLR4-active metabolite morphine-3 glucuronide sensitization of sensory neurons. Sci. Rep. 7, 3741 (2017).
Jung, K. et al. Toll-like receptor 4 decoy, TOY, attenuates gram-negative bacterial sepsis. PLOS ONE 4, e7403 (2009).
Lemonnier, E. et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl. Psychiatry 2, e202 (2012).
Lemonnier, E. & Ben-Ari, Y. The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr. 99, 1885–1888 (2010).
Lemonnier, E. et al. Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl. Psychiatry 7, e1056 (2017).
Pressler, R. M. et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 14, 469–477 (2015).
Sveinsdottir, S. et al. Altered expression of aquaporin 1 and 5 in the choroid plexus following preterm intraventricular hemorrhage. Developmental Neurosci. 36, 542–551 (2014).
Gharagozloo, M. et al. NLR-Dependent regulation of inflammation in multiple sclerosis. Front. Immunol. 8, 2012 (2017).
White, C. S., Lawrence, C. B., Brough, D. & Rivers-Auty, J. Inflammasomes as therapeutic targets for Alzheimer’s disease. Brain Pathol. 27, 223–234 (2017).
Ringstad, G., Vatnehol, S. A. S. & Eide, P. K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705 (2017).
Ringstad, G. et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3, 121537 (2018).
Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 340, 1529–1530 (2013).
Ding, Y. et al. Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp. Neurol. 320, 113003 (2019).
Plog, B. A. & Nedergaard, M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu. Rev. Pathol. 13, 379–394 (2018).
Jin, B. J., Smith, A. J. & Verkman, A. S. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol. 148, 489–501 (2016).
Smith, A. J., Yao, X., Dix, J. A., Jin, B. J. & Verkman, A. S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6, e27679 (2017).
Smith, A. J. & Verkman, A. S. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J. 32, 543–551 (2018).
Iliff, J. & Simon, M. CrossTalk proposal: the glymphatic system supports convective exchange of cerebrospinal fluid and brain interstitial fluid that is mediated by perivascular aquaporin-4. J. Physiol. 597, 4417–4419 (2019).
Smith, A. J. & Verkman, A. S. Rebuttal from Alex J. Smith and Alan S. Verkman. J. Physiol. 597, 4427–4428 (2019).
Simon, M. & Iliff, J. Rebuttal from Matthew Simon and Jeffrey Iliff. J. Physiol. 597, 4425–4426 (2019).
Eming, S. A., Hammerschmidt, M., Krieg, T. & Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 20, 517–527 (2009).
Klingberg, F., Hinz, B. & White, E. S. The myofibroblast matrix: implications for tissue repair and fibrosis. J. Pathol. 229, 298–309 (2013).
Sarnat, H. B. Ependymal reactions to injury. A review. J. Neuropathol. Exp. Neurol. 54, 1–15 (1995).
Fukumizu, M., Takashima, S. & Becker, L. E. Neonatal posthemorrhagic hydrocephalus: neuropathologic and immunohistochemical studies. Pediatric Neurol. 13, 230–234 (1995).
Marlin, A. E., Wald, A., Hochwald, G. M. & Malhan, C. Kaolin-induced hydrocephalus impairs CSF secretion by the choroid plexus. Neurology 28, 945–949 (1978).
Silverberg, G. D. et al. Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. J. Neurosurg. 97, 1271–1275 (2002).
Kosteljanetz, M. Cerebrospinal fluid production in subarachnoid haemorrhage. Br. J. Neurosurg. 2, 161–167 (1988).
Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9, 4878 (2018).
Wagshul, M. E., Eide, P. K. & Madsen, J. R. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 8, 5 (2011).
Hladky, S. B. & Barrand, M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26 (2014).
Shibukawa, S. et al. Time-spatial labeling inversion pulse (Time-SLIP) with pencil beam pulse: a selective labeling technique for observing cerebrospinal fluid flow dynamics. Magn. Reson. Med. Sci. 17, 259–264 (2018).
Yamada, S. et al. Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249, 644–652 (2008).
Hoffmann, A. et al. MRI of iron oxide nanoparticles and myeloperoxidase activity links inflammation to brain edema in experimental cerebral malaria. Radiology 290, 359–367 (2019).
Millward, J. M. et al. Application of europium-doped very small iron oxide nanoparticles to visualize neuroinflammation with MRI and fluorescence microscopy. Neuroscience 403, 136–144 (2017).
Acknowledgements
K.T.K. is supported by NIH grants NRCDP K12-228168, 1RO1NS109358 and R01 NS111029-01A1; the Hydrocephalus Association; the Rudy Schulte Research Institute; and the Simons Foundation. J.K.K. is supported by the Howard Hughes Medical Institute. M.N. is supported by NIH 1R01NS100366 and RF1 AG057575-01. P.Q.D. is supported by NIH Medical Scientist Training Program Grant T32GM007205. B.C.W. is supported by NIH grants 1R01HD096693 and 7R01HD085853 and NIH Director’s Pioneer Award 5DP1HD086071. S.J.S. is supported by NIH Director’s Pioneer Award, NIH Director’s Transformative Award 1R01AI145057, and NIH grants 1R01HD096693 and 7R01HD085853. D.D.L. is supported by NIH Director’s Pioneer Award 5DP1HD086071, the Patient-Centered Outcomes Research Institute (PCORI 1503–29700), the Hydrocephalus Association, and the Rudy Schulte Research Institute. D.D.L. also receives research support through Microbot Medical, Inc. J.M.S. is supported by grants from the Department of Veterans Affairs (I01BX002889), the Department of Defense (SCI170199), the National Heart, Lung and Blood Institute (R01HL082517), and the National Institute of Neurological Disorders and Stroke (R01NS060801, R01NS102589, R01NS105633). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Review criteria
We searched PubMed for articles in all year ranges with multiple combinations of search terms, including “post-haemorrhagic hydrocephalus”, “post-infectious hydrocephalus”, “worldwide”, “epidemiology”, “ETV/CPC”, “VP Shunt”, “NKCC1”, “SPAK”, “Toll-like receptors”, “inflammation”, “obstruction”, “impaired reabsorption”, “CSF hypersecretion”, “cerebrospinal fluid”. There were no language exclusions and articles chosen were based on relevance to topics covered in this Review.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, discussed the content of the article, wrote the text, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks M. Hamilton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Bulk flow model
-
Model of cerebrospinal fluid flow, in which cerebrospinal fluid moves from the choroid plexus through the cerebral ventricles and cisterns to the subarachnoid space, where reabsorption through the arachnoid granulations occurs.
- Neurohumoural mechanisms
-
Mechanisms involving the sympathetic nervous system and hormonal signalling.
- Periventricular heterotopia
-
Bilateral nodules of grey matter that line the lateral ventricles and consist of neurons that failed to migrate during fetal development.
- Time-spatial labelling inversion pulse imaging
-
A non-contrast MRI technique that uses cerebrospinal fluid as a tracer.
Rights and permissions
About this article
Cite this article
Karimy, J.K., Reeves, B.C., Damisah, E. et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol 16, 285–296 (2020). https://doi.org/10.1038/s41582-020-0321-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-0321-y
This article is cited by
-
Pro-inflammatory cerebrospinal fluid profile of neonates with intraventricular hemorrhage: clinical relevance and contrast with CNS infection
Fluids and Barriers of the CNS (2024)
-
Reduced racial disparities among newborns with intraventricular hemorrhage
Child's Nervous System (2024)
-
Comparative study between ventriculosubgaleal shunt and external ventricular drain for management of post infective hydrocephalus among pediatrics
Child's Nervous System (2024)
-
Outcomes of the 2019 hydrocephalus association workshop, "Driving common pathways: extending insights from posthemorrhagic hydrocephalus"
Fluids and Barriers of the CNS (2023)
-
Modelling idiopathic intracranial hypertension in rats: contributions of high fat diet and testosterone to intracranial pressure and cerebrospinal fluid production
Fluids and Barriers of the CNS (2023)