Abstract
Main conclusion
A hypothesis that squalene cyclase genes are widely distributed throughout ferns was proposed. We successfully isolated a squalene cyclase pseudogene from a fern from which no triterpene hydrocarbons were detected
Abstract
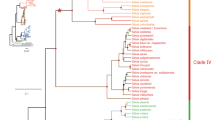
Ferns are the most primitive vascular plants, with their locations ranging from tropical to cold temperate regions and from lowland to alpine zones. The triterpene hydrocarbons and their derivatives are characteristic fern metabolites, and are also chemophenetic markers. Recently, our biosynthetic study into fern squalene cyclases (SCs), the enzymes responsible for triterpene synthesis, gave an unexpected inconsistency between genotype (enzyme function) and chemotype (triterpene profile). This finding prompted us to propose a hypothesis that SC genes are widely distributed throughout ferns and lycophytes whether or not they produce triterpene hydrocarbons. To test this hypothesis, we employed a multifaceted approach based on phytochemical, biochemical, and phylogenetic analyses. As anticipated, we successfully isolated two SC pseudogenes from a fern from in which no or only one triterpene hydrocarbon was detected. Subsequent mutagenesis experiments resulted in the functional conversion of these pseudogenes into active SC genes. Given an auxiliary hypothesis regarding the inherent limit of the degenerate polymerase chain reaction (PCR) method, the overall dataset supported our hypothesis, although correction was required with respect to plant coverage. Not only did the corrected hypothesis outline the distribution of SC genes throughout ferns, it provided insight into the molecular basis of the triterpene-based chemophenetics in ferns, which is also discussed.





Similar content being viewed by others
Abbreviations
- cDNA :
-
Complementary DNA
- SC :
-
Squalene cyclase
References
Ageta H, Masuda K, Inoue M, Ishida T (1982) Fern constituent: Colysanoxide, an onoceroid having a novel carbon skeleton, isolated from Colysis species. Tetrahedron Lett 23:4349–4352
Ageta H, Shiojima K, Arai Y, Suzuki H, Kiyotani T (1994) NMR-spectra of triterpenoids 2. Hopenes and migrated hopenes. Chem Pharm Bull 42:39–44
Ageta H, Shiojima K, Arai Y, Masuda K (1998) Chmotaxonomy of ferns. In: Ageta H, Aimi N, Ebizuka Y, Fujita T, Honda G (eds) Towards natural medicine research in the 21st century: proceedings of the international symposium on natural medicines, October 28–30, 1997 Kyoto, Japan. Excerpta Medica, Amsterdam, pp 3–13.
Araki T, Saga Y, Marugami M, Otaka J, Araya H, Saito K, Yamazaki M, Suzuki H, Kushiro T (2016) Onocerin biosynthesis requires two highly dedicated triterpene cyclases in a fern Lycopodium clavatum. ChemBioChem 17:288–290
Asakawa Y, Ludwiczuk A, Nagashima F (2013) Progress in the chemistry of organic natural products, vol 95. Springer, Cham
Banks JA, Nishiyama T, Hasebe M et al (2011) The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332:960–963
CBOL Plant Working Group (2009) A DNA barcode for land plants. Proc Natl Acad Sci USA 106:12794–12797
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Kress WJ, Erickson DL (2007) A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2:e508
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Li FW, Brouwer P, Carretero-Paulet L et al (2018) Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat Plants 4:460–472
Lodeiro S, Xiong Q, Wilson WK, Kolesnikova MD, Onak CS, Matsuda SPT (2007) An oxidosqualene cyclase makes numerous products by diverse mechanisms: a challenge to prevailing concepts of triterpene biosynthesis. J Am Chem Soc 129:11213–11222
Murakami T, Tanaka N (1988) Progress in the chemistry of organic natural products, vol 54. Springer, Cham
Saga Y, Araki T, Araya H, Saito K, Yamazaki M, Suzuki H, Kushiro T (2017) Identification of serratane synthase gene from the fern Lycopodium clavatum. Org Lett 19:496–499
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakakibara J, Watanabe R, Kanai Y, Ono T (1995) Molecular cloning and expression of rat squalene epoxidase. J Biol Chem 270:17–20
Shinozaki J, Shibuya M, Masuda K, Ebizuka Y (2008a) Squalene cyclase and oxidosqualene cyclase from a fern. FEBS Lett 582:310–318
Shinozaki J, Shibuya M, Masuda K, Ebizuka Y (2008b) Dammaradiene synthase, a squalene cyclase, from Dryopteris crassirhizoma Nakai. Phytochemistry 69:2559–2564
Shinozaki J, Shibuya M, Takahata Y, Masuda K, Ebizuka Y (2010) Molecular evolution of fern squalene cyclases. ChemBioChem 11:426–433
Shinozaki J, Hiruta M, Okada T, Masuda K (2016) Migrated hopene synthases from Colysis pothifolia and identification of a migration switch controlling the number of 1,2-hydride and methyl shifts. ChemBioChem 17:65–70
Shinozaki J, Nakene T, Takano A (2018) Squalene cyclases and cycloartenol synthases from Polystichum polyblepharum and six allied ferns. Molecules 23:1843
Shiojima K, Arai Y, Masuda K, Kamata T, Suzuki M, Ageta H (1997) Chemotaxonomy of fern plants (V) Japanese Polystichum species. Nat Med 51:523–527
Siedenburg G, Jendrossek D (2011) Squalene-hopene cyclases. Appl Environ Microbiol 77:3905–3915
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Zidorn C (2019) Plant chemophenetics—a new term for plant chemosystematics/plant chemotaxonomy in the macro-molecular era. Phytochemistry 163:147–148
Acknowledgements
We wish to thank Dr. K. Masuda for providing suggestions on triterpene-based chemotaxonomy in ferns. We thank Y. Takase and K. Chiba for their assistance in GC–MS measurements. We would also like to thank Editage (https://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shinozaki, J., Ohtake, M., Sato, M. et al. Molecular basis of triterpene-based chemophenetics in ferns. Planta 251, 78 (2020). https://doi.org/10.1007/s00425-020-03369-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03369-3