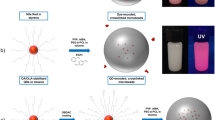
Abstract
A one-step synthesis using the reversed-phase suspension polymerization method and ultraviolet light curing is proposed for preparing the Raman-encoded suspension array (SA). The encoded microcarriers are prepared by doping the Raman reporter molecules into an aqueous phase, and then dispersing the aqueous phase in an oil phase and curing by ultraviolet light irradiation. The multiplexed biomolecule detection and various concentration experiments confirm the qualitative and quantitative analysis capabilities of the Raman-encoded SA with a limit of detection of 52.68 pM. The narrow bandwidth of the Raman spectrum can achieve a large number of codes in the available spectral range and the independence between the encoding channel and the fluorescent label channel provides the encoding method with high accuracy. This preparation method is simple and easy to operate, low in cost, and high in efficiency. A large number of hydrogel-based encoding microbeads could be quickly obtained with good biocompatibility. Most importantly, concentrating plenty of Raman reporter molecules inside the microbeads increases the signal intensity and means the molecular assembly is not limited by the functional groups; thus, the types of materials available for Raman encoding method are expanded. Furthermore, the signal intensity–related encoding method is verified by doping different proportions of Raman reporter molecules with our proposed synthesis method, which further increases the detection throughput of Raman-encoded SA.

Graphical Abstract







Similar content being viewed by others
References
Robert W, Cossins AR, Spiller DG. Encoded microcarriers for high-throughput multiplexed detection. Angew Chem Int Ed. 2007;45(37):6104–17.
Lutz BR, Dentinger CE, Nguyen LN, Lei S, Jingwu Z, Allen AN, et al. Spectral analysis of multiplex Raman probe signatures. ACS Nano. 2008;2(11):2306.
Zou F, Zhou H, Kim J, Koh K, Lee J. Dual-mode SERS-fluorescence immunoassay using graphene quantum dot labeling on one-dimensional aligned magnetoplasmonic nanoparticles. ACS Appl Mater Interfaces. 2015;7(22):12168–75.
Wang F, Deng R, Wang J, Wang Q, Han Y, Zhu H, et al. Tuning upconversion through energy migration in core–shell nanoparticles. Nat Mater. 2011;10:968. https://www.nature.com/articles/nmat3149#supplementary-information. https://doi.org/10.1038/nmat3149.
Gang W, Yuankui L, Hongjing D, Lu W, Wanwan L, Xiebing W, et al. Highly efficient preparation of multiscaled quantum dot barcodes for multiplexed hepatitis B detection. ACS Nano. 2013;7(1):471.
Lee H, Lee D, Park JH, Song SH, Jeong IG, Kim CS, et al. High throughput differential identification of TMPRSS2-ERG fusion genes in prostate cancer patient urine. Biomaterials. 2017;135:23–9.
Yi Y, Wei L, Peng S, Rui L, Li Y, Xu J, et al. Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array. Sensors Actuators B Chem. 2017;248:351–8.
Lu B, He Q, He Y, Chen X, Feng G, Liu S, et al. Dual-channel-coded microbeads for multiplexed detection of biomolecules using assembling of quantum dots and element coding nanoparticles. Anal Chim Acta. 2018;1024:153–60. https://doi.org/10.1016/j.aca.2018.03.025.
Cao D, Li CY, Qi CB, Chen HL, Pang DW, Tang HW. Multiple optical trapping assisted bead-array based fluorescence assay of free and total prostate-specific antigen in serum. Sensors Actuators B Chem. 2018;269:143–50.
Lim CT, Zhang Y. Bead-based microfluidic immunoassays: the next generation. Biosens Bioelectron. 2007;22(7):1197–204.
Zhao Y, Zhao X, Pei X, Hu J, Zhao W, Chen B, et al. Multiplex detection of tumor markers with photonic suspension array. Anal Chim Acta. 2009;633(1):103–8.
Vaidya SV, M Lane G, Charles M, Alexander C. Spectral bar coding of polystyrene microbeads using multicolored quantum dots. Anal Chem. 2007;79(22):8520–30.
Supratim G, Sykes EA, Jennings TL, Chan WCW. Rapid screening of genetic biomarkers of infectious agents using quantum dot barcodes. ACS Nano. 2011;5(3):1580–7.
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–44.
Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19(7):631–5.
Feng G, He Q, Xie W, He Y, Chen X, Wang B, et al. Dual-spectra encoded suspension array using reversed-phase microemulsion UV curing and electrostatic self-assembling. RSC Adv. 2018;8(38):21272–9. https://doi.org/10.1039/C8RA02410C.
Mercato LL, Del, Abbasi AZ, Markus O, Parak WJ. Multiplexed sensing of ions with barcoded polyelectrolyte capsules. ACS Nano. 2011;5(12):9668–74.
Shen Z, He Y, Zhang G, He Q, Li D, Ji Y. Dual-wavelength digital holographic phase and fluorescence microscopy for an optical thickness encoded suspension array. Opt Lett. 2018;43(4):739–42. https://doi.org/10.1364/OL.43.000739.
Yunwei Charles C, Rongchao J, Mirkin CA. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science. 2002;297(5586):1536–40.
Keisham B, Cole A, Nguyen P, Mehta A, Berry V. Cancer cell hyperactivity and membrane dipolarity monitoring via Raman mapping of interfaced graphene: toward non-invasive cancer diagnostics. ACS Appl Mater Interfaces. 2016;8(48):32717–22. https://doi.org/10.1021/acsami.6b12307.
Li JM, Wei C, Ma WF, An Q, Guo J, Hu J, et al. Multiplexed SERS detection of DNA targets in a sandwich-hybridization assay using SERS-encoded core–shell nanospheres. J Mater Chem. 2012;22(24):12100–6.
Zong S, Wang Z, Zhang R, Wang C, Xu S, Cui Y. A multiplex and straightforward aqueous phase immunoassay protocol through the combination of SERS-fluorescence dual mode nanoprobes and magnetic nanobeads. Biosens Bioelectron. 2013;41(1):745–51.
Kim J, Maa M, Zagorovsky K, Chan W. State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials. 2017;146:97.
Li R, Zhang Y, Tan J, Wan J, Guo J, Wang C. Dual-mode encoded magnetic composite microsphere based on fluorescence reporters and Raman probes as covert tag for anticounterfeiting applications. ACS Appl Mater Interfaces. 2016;8(14):9384–94.
Zhuyuan W, Shenfei Z, Wang L, Chunlei W, Shuhong X, Hui C, et al. SERS-fluorescence joint spectral encoding using organic-metal-QD hybrid nanoparticles with a huge encoding capacity for high-throughput biodetection: putting theory into practice. J Am Chem Soc. 2012;134(6):2993–3000.
Hongmei L, Xinping Z, Tianrui Z. Plasmonic nano-ring arrays through patterning gold nanoparticles into interferograms. Opt Express. 2013;21(13):15314–22.
Jun BH, Kim JH, Park H, Kim JS, Yu KN, Lee SM, et al. Surface-enhanced Raman spectroscopic-encoded beads for multiplex immunoassay. J Comb Chem. 2007;9(2):237.
Nidhi N, Ashutosh C. A colorimetric gold nanoparticle sensor to interrogate biomolecular interactions in real time on a surface. Anal Chem. 2002;74(3):504–9.
Freeman RG, Grabar KC, Allison KJ, Bright RM, Davis JA, Guthrie AP, et al. Self-assembled metal colloid monolayers: an approach to SERS substrates. Science. 1995;267(5204):1629–32.
Michael H, Johan R, Dieter S, Templin MF, Joos TO. Protein microarrays for diagnostic assays. Anal Bioanal Chem. 2009;393(5):1407–16.
Wang B, Guan T, Jiang J, He Q, Chen X, Feng G, et al. Gold-nanorod-enhanced Raman spectroscopy encoded micro-quartz pieces for the multiplex detection of biomolecules. Anal Bioanal Chem. 2019;411(21):5509–18. https://doi.org/10.1007/s00216-019-01929-5.
Lai Y, Sun S, He T, Schlücker S, Wang Y. Raman-encoded microbeads for spectral multiplexing with SERS detection. RSC Adv. 2015;5(18):13762–7.
You L, Li R, Dong X, Wang F, Guo J, Wang C. Micron-sized surface enhanced Raman scattering reporter/fluorescence probe encoded colloidal microspheres for sensitive DNA detection. J Colloid Interface Sci. 2017;488:109–17.
Wang X, Jiang Y, Wang YW, Huang MT, Ho CT, Huang Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108(2):419–24.
Feng G, Guan T, He Q, Lu B, Chen X, Wang B, et al. Ion-chelation based digital barcodes for multiplexing of a suspension array. Analyst. 2019;144(13):4093–9.
Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater. 2011;7(6):2467–76.
Stephanie N, Hayenga HN, West JL. PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnol Bioeng. 2010;105(4):636–44.
Shohatee D, Keifer J, Schimmel N, Mohanty S, Ghosh G. Hydrogel-based suspension array for biomarker detection using horseradish peroxidase-mediated silver precipitation. Anal Chim Acta. 2018;999(25):132–8. https://doi.org/10.1016/j.aca.2017.10.033.
Haeshin L, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–30.
Liu Y, Liu L, He Y, He Q, Ma H. Quantum-dots-encoded-microbeads based molecularly imprinted polymer. Biosens Bioelectron. 2016;77:886–93.
He Q, Chen X, He Y, Guan T, Feng G, Lu B, et al. Spectral-optical-tweezer-assisted fluorescence multiplexing system for QDs-encoded bead-array bioassay. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2019.01.004.
Funding
This research was made possible with the financial support from the National Science Foundation of China (NSFC) (61875102, 81871395, 61675113), the Science and Technology Research Program of Shenzhen City (JCYJ20170816161836562, JCYJ20170817111912585, JCYJ20160427183803458, JCYJ20170412171856582, JCY20180508152528735), the Oversea Cooperation Foundation, and the Graduate School at Shenzhen, Tsinghua University (HW2018007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 230 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Zhou, X., He, Q. et al. Hydrogel-based microbeads for Raman-encoded suspension array using the reversed-phase suspension polymerization method and ultraviolet light curing. Anal Bioanal Chem 412, 2731–2741 (2020). https://doi.org/10.1007/s00216-020-02528-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02528-5