Abstract
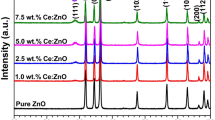
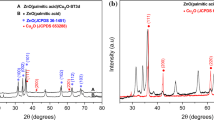
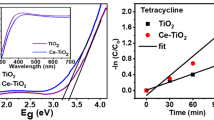
In this study, we successfully synthesized ZnO/CeO2 composite nanoparticles for efficient ultraviolet (UV) filtering applications using a simple precipitation route. Various ratios of Ce/Ti, 2.5 at.%, 5 at.%, and 10 at.% were used to precipitate ceria onto commercial ZnO nanopowder at pH 9. The calculated mean crystallite sizes of the resultant nanocomposites were ~ 90 nm, ~ 79 nm, and ~ 41 nm for the 2.5 at.%, 5 at.% and 10 at.% ceria amounts, respectively. A stronger and more selective absorbance within the UV range was observed due to precipitation of a small amount of ceria to decorate the commercial ZnO surface. The photocatalyst results show that the addition of ceria, particularly with the precipitation amount increased up to 10 at.%, can effectively reduce crystal violet degradation by about 97% in a period of time from 0 to 30 min when exposed to ultraviolet light over 30 min or by around 99% under solar simulation for 30 min.












Similar content being viewed by others
References
Furusawa T et al (2008) The microwave effect on the properties of silica-coated TiO2 fine particles prepared using sol–gel method. Mater Res Bull 43(4):946–957
Kullavanijaya P, Lim HW (2005) Photoprotection. J Am Acad Dermatol 52(6):937–958
Liu X, Yin S, Sato T (2009) Synthesis of broad-spectrum UV-shielding plate-like titanate/calcia-doped ceria composite in different pH solution. Mater Chem Phys 116(2–3):421–425
Antoniou C et al (2008) Sunscreens–what’s important to know. J Eur Acad Dermatol Venereol 22(9):1110–1119
Im YM et al (2015) Effect of ZnO nanoparticles morphology on UV blocking of poly (vinyl alcohol)/ZnO composite nanofibers. Mater Lett 147:20–24
Pinnell SR et al (2000) Microfine zinc oxide is a superior sunscreen ingredient to microfine titanium dioxide. Dermatol Surg 26(4):309–314
Schaefer H, Moyal D, Fourtanier A (1998) Recent advances in sun protection. Protection of the skin against ultraviolet radiations. John Libbey Eurotext, Paris, pp 119–129
Schauder S, Ippen H (1997) Contact and photocontact sensitivity to sunscreens: review of a 15-year experience and of the literature. Contact Dermat 37(5):221–232
Roscher NM et al (1994) Photodecomposition of several compounds commonly used as sunscreen agents. J Photochem Photobiol, A 80(1–3):417–421
Serpone N et al (2002) An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents. Photochem Photobiol Sci 1(12):970–981
Dodd A et al (2010) Optical and photocatalytic properties of nanoparticulate (TiO2) x (ZnO) 1–x powders. J Alloy Compd 489(2):L17–L21
Cai R et al (1991) Photokilling of malignant cells with ultrafine TiO2 powder. Bull Chem Soc Japan 64(4):1268–1273
Serpone N, Salinaro A, Emeline A (2001) Deleterious effects of sunscreen titanium dioxide nanoparticles on DNA: efforts to limit DNA damage by particle surface modification. In: Nanoparticles and nanostructured surfaces: novel reporters with biological applications. 2001. International society for optics and photonics
Yang H, Zhu S, Pan N (2004) Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J Appl Polym Sci 92(5):3201–3210
Chen H-C et al (2006) Effects of temperature on columnar microstructure and recrystallization of TiO2 film produced by ion-assisted deposition. Appl Opt 45(9):1979–1984
Senatova S et al (2015) Optical properties of stabilized ZnO nanoparticles, perspective for UV-protection in sunscreens. Curr Nanosci 11(3):354–359
Zholobak N et al (2011) UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J Photochem Photobiol, B 102(1):32–38
Boutard T et al (2013) Comparison of photoprotection efficiency and antiproliferative activity of ZnO commercial sunscreens and CeO2. Mater Lett 108:13–16
Yabe S, Sato T (2003) Cerium oxide for sunscreen cosmetics. J Solid State Chem 171(1–2):7–11
Truffault L et al (2010) Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater Res Bull 45(5):527–535
Truffault L et al (2011) Synthesis and characterization of Fe doped CeO. Nanosci Nanotechnol 11:1–10
Truffault L et al (2011) Synthesis of nano-hematite for possible use in sunscreens. J Nanosci Nanotechnol 11(3):2413–2420
Cardillo D, Konstantinov K, Devers T (2013) The effects of cerium doping on the size, morphology, and optical properties of α-hematite nanoparticles for ultraviolet filtration. Mater Res Bull 48(11):4521–4525
Heckert EG et al (2008) The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29(18):2705–2709
Celardo I et al (2011) Ce3 + ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 5(6):4537–4549
He G, Fan H, Wang Z (2014) Enhanced optical properties of heterostructured ZnO/CeO2 nanocomposite fabricated by one-pot hydrothermal method: fluorescence and ultraviolet absorption and visible light transparency. Opt Mater 38:145–153
Li R et al (2002) UV-shielding properties of zinc oxide-doped ceria fine powders derived via soft solution chemical routes. Mater Chem Phys 75(1–3):39–44
Yabe S et al (2001) Synthesis and UV-shielding properties of metal oxide doped ceria via soft solution chemical processes. Int J Inorg Mater 3(7):1003–1008
Bi L et al (2008) Structural, magnetic, and magneto-optical properties of Co-doped Ce O2−δ films. J Appl Phys 103(7):07D138
He Y, Yang B, Cheng G (2003) Controlled synthesis of CeO2 nanoparticles from the coupling route of homogenous precipitation with microemulsion. Mater Lett 57(13–14):1880–1884
Yamashita M et al (2002) Synthesis and microstructure of calcia doped ceria as UV filters. J Mater Sci 37(4):683–687. https://doi.org/10.1023/A:1013819310041
Ge C, Xie C, Cai S (2007) Preparation and gas-sensing properties of Ce-doped ZnO thin-film sensors by dip-coating. Mater Sci Eng, B 137(1–3):53–58
Li C et al (2011) Electrospinning of CeO2–ZnO composite nanofibers and their photocatalytic property. Mater Lett 65(9):1327–1330
Yayapao O et al (2013) Sonochemical synthesis, photocatalysis and photonic properties of 3% Ce-doped ZnO nanoneedles. Ceram Int 39:S563–S568
Yousefi M et al (2011) Enhanced photoelectrochemical activity of Ce doped ZnO nanocomposite thin films under visible light. J Electroanal Chem 661(1):106–112
Fangli D et al (2010) Preparation, characterization and infrared emissivity study of Ce-doped ZnO films. J Rare Earths 28(3):391–395
Anbia M, Fard SEM (2012) Humidity sensing properties of Ce-doped nanoporous ZnO thin film prepared by sol–gel method. J Rare Earths 30(1):38–42
Morinaga Y et al (1997) Effect of Ce doping on the growth of ZnO thin films. J Cryst Growth 174(1–4):691–695
Mahmoud WE (2010) Synthesis and optical properties of Ce-doped ZnO hexagonal nanoplatelets. J Cryst Growth 312(21):3075–3079
de Lima JF et al (2009) ZnO: CeO2-based nanopowders with low catalytic activity as UV absorbers. Appl Surf Sci 255(22):9006–9009
Panda N et al (2013) Thermoluminescence and decay studies on cerium doped ZnO nanopowders. Mater Lett 95:205–208
Yang J et al (2008) Low-temperature growth and optical properties of Ce-doped ZnO nanorods. Appl Surf Sci 255(5):2646–2650
Dar G et al (2012) Ce-doped ZnO nanorods for the detection of hazardous chemical. Sens Actuators B Chem 173:72–78
Tan WK et al (2013) Photoluminescence properties of rod-like Ce-doped ZnO nanostructured films formed by hot-water treatment of sol–gel derived coating. Opt Mater 35(11):1902–1907
Rezaei M, Habibi-Yangjeh A (2013) Simple and large scale refluxing method for preparation of Ce-doped ZnO nanostructures as highly efficient photocatalyst. Appl Surf Sci 265:591–596
Xia C, Hu C, Zhou P (2013) Low-temperature growth and optical properties of Ce-doped ZnO nanorods. J Exp Nanosci 8(1):69–76
Sofiani Z et al (2006) Optical properties of ZnO and ZnO: Ce layers grown by spray pyrolysis. Opt Commun 267(2):433–439
George A et al (2011) Detailed of X-ray diffraction and photoluminescence studies of Ce doped ZnO nanocrystals. J Alloy Compd 509(20):5942–5946
Karunakaran C, Gomathisankar P, Manikandan G (2010) Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater Chem Phys 123(2–3):585–594
Bogusz K et al (2018) TiO2/(BiO)2 CO3 nanocomposites for ultraviolet filtration with reduced photocatalytic activity. J Mater Chem C 6(21):5639–5650
Cardillo D et al (2016) Multifunctional Fe2 O3/CeO2 nanocomposites for free radical scavenging ultraviolet protection. RSC Adv 6(70):65397–65402
Rajendran S et al (2016) Ce 3 + -ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci Rep 6:31641
Saravanan R et al (2018) Line defect Ce3 + induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J Photochem Photobiol, A 353:499–506
Saravanan R, et al. (2012) Photocatalytic degradation of organic dyes using ZnO/CeO2 nanocomposite material under visible light. In Advanced materials research. Trans Tech Publications Ltd
Ying JY, Tschöpe A (1996) Synthesis and characteristics of non-stoichiometric nanocrystalline cerium oxide-based catalysts. Chem Eng J Biochem Eng J 64(2):225–237
Nelson BC et al (2016) Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 5(2):15
Xue Y et al (2011) Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C 115(11):4433–4438
Lamba R et al (2015) CeO2ZnO hexagonal nanodisks: efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar light. J Alloy Compd 620:67–73
Ye Z et al (2016) Well-dispersed nebula-like ZnO/CeO2@ HNTs heterostructure for efficient photocatalytic degradation of tetracycline. Chem Eng J 304:917–933
Zamiri R et al (2015) Dielectrical properties of CeO2 nanoparticles at different temperatures. PLoS ONE 10(4):e0131851
Zuas O, Abimanyu H, Wibowo W (2014) Synthesis and characterization of nanostructured CeO2 with dyes adsorption property. Process Appl Ceram 8(1):39–46
Kumar E, Selvarajan P, Muthuraj D (2013) Synthesis and characterization of CeO2 nanocrystals by solvothermal route. Mater Res 16(2):269–276
Nagaraju G et al (2017) Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater Res Bull 94:54–63
Selvi N, Sankar S, Dinakaran K (2014) Size controlled synthesis of pure CeO2 and ZnO COATED CeO2 core-shell nanoparicles for opto-electronic applications. In: 2014 International conference on science engineering and management research (ICSEMR). IEEE
Selvi N et al (2014) Effect of ZnO, SiO2 dual shells on CeO2 hybrid core–shell nanostructures and their structural, optical and magnetic properties. RSC Adv 4(99):55745–55751
Suhail FSA, Mashkour MS, Saeb D (2015) The study on photo degradation of crystal violet by polarographic technique. Int J Basic Appl Sci 15:12–21
Lee G, Kawazoe T, Ohtsu M (2002) Difference in optical bandgap between zinc-blende and wurtzite ZnO structure formed on sapphire (0001) substrate. Solid State Commun 124(5–6):163–165
Mueen R et al (2020) Na-doped ZnO UV filters with reduced photocatalytic activity for sunscreen applications. J Mater Sci 55(7):2772–2786. https://doi.org/10.1007/s10853-019-04122-2
Tsuzuki T et al (2012) Reduction of the photocatalytic activity of ZnO nanoparticles for UV protection applications. Int J Nanotechnol 9(10–12):1017–1029
He R, Hocking RK, Tsuzuki T (2012) Co-doped ZnO nanopowders: location of cobalt and reduction in photocatalytic activity. Mater Chem Phys 132(2–3):1035–1040
Kaneva NV, Dimitrov DT, Dushkin CD (2011) Effect of nickel doping on the photocatalytic activity of ZnO thin films under UV and visible light. Appl Surf Sci 257(18):8113–8120
Acknowledgements
This work is part of the University of Wollongong Global Challenges project “NEXT GENERATION SUNSCREENS: Designed and tested for Australian conditions, with global implications for sun safety.” Furthermore, the authors acknowledge the use of the facilities within the Electron Microscopy Centre at the University of Wollongong. The authors would also like to acknowledge the support provided by the University of Diyala and the Iraqi Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mueen, R., Morlando, A., Qutaish, H. et al. ZnO/CeO2 nanocomposite with low photocatalytic activity as efficient UV filters. J Mater Sci 55, 6834–6847 (2020). https://doi.org/10.1007/s10853-020-04493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04493-x