Abstract
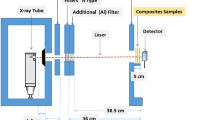
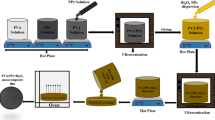
The aim of this research is to present a method to evaluate the X-ray attenuation coefficient of (nano)particles by uniformly dispersing them in a host polymer, polydimethylsiloxane. The method is described and applied to the case of a material of interest for medical application in X-ray shielding and imaging, namely bismuth oxychloride nanoplates (BiOCl NP). A diagnostic X-ray machine was used to perform X-ray attenuation tests. A certified copper plate was used for calibration purposes and to find the X-ray photon energy. Blank polymer and composites were investigated, and the attenuation coefficient μ and the mass attenuation coefficient μ/ρ of BiOCl NPs were found to be 45 cm−1 and 6 cm2 g−1, respectively. Based on these values, a discussion is presented in which the perspective applications of BiOCl in medicine are discussed with specific focus on (i) X-ray shielding and (ii) X-ray imaging.





Similar content being viewed by others
References
Studwell AJ, Kotton DN (2011) A shift from cell cultures to creatures. in vivo imaging of small animals in experimental regenerative medicine. Mol Ther 19:1933–1941. https://doi.org/10.1038/mt.2011.194
Zagorchev L, Oses P, Zhuang ZW et al (2010) Micro computed tomography for vascular exploration. J Angiogenes Res 2:7. https://doi.org/10.1186/2040-2384-2-7
Griffiths JR, Glickson JD (2000) Monitoring pharmacokinetics of anticancer drugs: non-invasive investigation using magnetic resonance spectroscopy. Adv Drug Deliv Rev 41:75–89. https://doi.org/10.1016/S0169-409X(99)00057-5
Salem KA, Szymanski-Exner A, Lazebnik RS, Breen MS, Gao J, Wilson DL (2002) X-ray computed tomography methods for in vivo evaluation of local drug release systems. IEEE Trans Med Imaging 21:1310–1316
Lyra M, Charalambatou P, Sotiropoulos MDS (2011) Radiation protection of staff in 111In radionuclide therapy is the lead apron shielding effective? Radiat Prot Dosim 147:272–276. https://doi.org/10.1093/rpd/ncr330
Schueler BA (2010) Operator Shielding: How and Why. Tech Vasc Interv Radiol 13:167–171. https://doi.org/10.1053/j.tvir.2010.03.005
Chida K, Kato M, Kagaya Y et al (2010) Radiation dose and radiation protection for patients and physicians during interventional procedure. J Radiat Res 51:97–105. https://doi.org/10.1269/jrr.09112
Kennedy EV, Iball GR, Brettle DS (2007) Investigation into the effects of lead shielding for fetal dose reduction in CT pulmonary angiography. Br J Radiol 80:631–638. https://doi.org/10.1259/bjr/31771954
Aral N, Banu Nergis F, Candan C (2016) An alternative X-ray shielding material based on coated textiles. Text Res J 86:803–811. https://doi.org/10.1177/0040517515590409
Botelho MZ, Künzel R, Okuno E et al (2011) X-ray transmission through nanostructured and microstructured CuO materials. Appl Radiat Isot 69:527–530. https://doi.org/10.1016/j.apradiso.2010.11.002
Weissleder R, Ntziachristos V (2003) Shedding light onto live molecular targets. Nat Med 9:123–128
Ntziachristos V, Ripoll J, Wang LV, Weissleder R (2005) Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol 23:313–320
Fa J, Weissleder R (2005) Molecular imaging in the clinical arena. JAMA 293:855–862
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171
Whitesides GM (2003) The “right” size in nanobiotechnology. Nat Biotechnol 21:1161–1165
Park JW (2002) Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res 4:95–99. https://doi.org/10.1186/bcr432
Kircher MF, Mahmood U, King RS et al (2003) A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res 63:8122–8125
Neuwelt EA, Várallyay P, Bagó AG, Muldoon LL, Nesbit G, Nixon R (2004) Imaging of iron oxide nanoparticles with MR and light microscopy in patients with malignant brain tumors. Neuropathol Appl Neurobiol 30:456–471
Nam J-M, Stoeva SI, Mirkin CA (2004) Bio-bar-code-based DNA detection with PCR-like sensitivity. J Am Chem Soc 126:5932–5933. https://doi.org/10.1021/ja049384+
Rabin O, Manuel Perez J, Grimm J et al (2006) An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater 5:118–122
Hainfeld JF, Slatkin DN, Smilowitz HM (2004) The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 49:N309–N315
Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM (2006) Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 79:248–253. https://doi.org/10.1259/bjr/13169882
Ross RD, Cole LE, Tilley JMR, Roeder RK (2014) Effects of functionalized gold nanoparticle size on X-ray attenuation and substrate binding affinity. Chem Mater 26:1187–1194. https://doi.org/10.1021/cm4035616
Burgess AE (1985) Physical measurements of heavy metal filter performance. Med Phys 12:225–228. https://doi.org/10.1118/1.595709
Aljundi T, Jensen T, Gray JN (1996) Heavy metal contamination detection using X-ray. In: Thompson DO, Chimenti DE (eds) Review of progress in quantitative nondestructive evaluation, vol15A. Plenum Press, New York, pp 465–472
Brenner DJ, Hall EJ (2007) Computed Tomography — An Increasing Source of Radiation Exposure. N Engl J Med 357:2277–2284. https://doi.org/10.1056/NEJMra072149
Kim SC, Lee HK, Cho JH (2014) Analysis of low-dose radiation shield effectiveness of multi-gate polymeric sheets. Radiat Eff Defects Solids 169:584–591. https://doi.org/10.1080/10420150.2014.920019
Needleman H (2004) Lead poisoning. Annu Rev Med 55:209–222
Flegal DRS, Flegal AR (1995) Lead in the biosphere: recent trends. Ambio 24:21–23
Pfeiffer F, Weitkamp T, Bunk O, David C (2006) Phase retrieval and differential phase-contrast imaging with low-brilliance X-ray sources. Nat Phys 2:258–261
Zanette I, Weitkamp T, Donath T et al (2010) Two-dimensional X-ray grating interferometer. Phys Rev Lett 105:248102. https://doi.org/10.1103/PhysRevLett.105.248102
Hammer B, Norskov JK (2002) Why gold is the noblest of all the metals. Nature 376:238–240
Kandanapitiye MS, Gao M, Molter J et al (2014) Synthesis, characterization, and X-ray attenuation properties of ultrasmall BiOI nanoparticles: toward renal clearable particulate CT contrast agents. Inorg Chem 53:10189–10194. https://doi.org/10.1021/ic5011709
Maghrabi HA, Vijayan A, Deb P, Wang L (2016) Bismuth oxide-coated fabrics for X-ray shielding. Text Res J 86:649–658. https://doi.org/10.1177/0040517515592809
Lei Y, Du Y, Li J et al (2014) Fabrication of X-ray absorption gratings via micro-casting for grating-based phase contrast imaging. J Micromech Microeng 24:015007. https://doi.org/10.1088/0960-1317/24/1/015007
Lei Y, Du Y, Li J et al (2013) Application of Bi absorption gratings in grating-based X-ray phase contrast imaging. Appl Phys Express 6:117301. https://doi.org/10.7567/APEX.6.117301
Nambiar S, Osei EK, Yeow JT (2014) Polymer nanocomposite-based shielding against diagnostic X-rays. J Appl Polym Sci 131:4939–4946. https://doi.org/10.1002/app.37980
Abdel-Aziz MM, Badran AS, Abdel-Hakem AA et al (1991) Styrene–butadiene rubber/lead oxide composites as gamma radiation shields. J Appl Polym Sci 42:1073–1080. https://doi.org/10.1002/app.1991.070420420
Harish V, Nagaiah N, Prabhu TN, Varughese KT (2009) Preparation and characterization of lead monoxide filled unsaturated polyester based polymer composites for gamma radiation shielding applications. J Appl Polym Sci 112:1503–1508. https://doi.org/10.1002/app.29633
Kean S (2011) The disappearing spoon (and other true tales of madness, love, and the history of the world from the periodic table of elements). Back Bay Books, New York
Sadler PJ, Li H, Sun H (1999) Coordination chemistry of metals in medicine: target sites for bismuth. Coord Chem Rev 185:689–709. https://doi.org/10.1016/S0010-8545(99)00018-1
Slikkerveer A, de Wolff FA (1989) Pharmacokinetics and toxicity of bismuth compounds. Med Toxicol Adverse Drug Exp 4:303–323. https://doi.org/10.1007/BF03259915
Sun H, Sadler PJ (1999) Bismuth antiulcer complexes. In: Clarke MJ, Sadler PJ (eds) Metallopharmaceuticals II: diagnosis and therapy. Springer, Berlin, pp 159–185
Suzuki H, Komatsu N, Ogawa T, Murafuji T, Ikegami T, Matano Y (2001) Organobismuth chemistry. Elsevier, Amsterdam
Briand GG, Burford N (1999) Bismuth compounds and preparations with biological or medicinal relevance. Chem Rev 99:2601–2658. https://doi.org/10.1021/cr980425s
Gao X, Zhang X, Wang Y et al (2015) An in vitro study on the cytotoxicity of bismuth oxychloride nanosheets in human HaCaT keratinocytes. Food Chem Toxicol 80:52–61. https://doi.org/10.1016/j.fct.2015.02.018
Li Y, Song Z, Li C et al (2013) Efficient near-infrared to visible and ultraviolet upconversion in polycrystalline BiOCl:Er3+/Yb3+ synthesized at low temperature. Ceram Int 39:8911–8916. https://doi.org/10.1016/j.ceramint.2013.04.085
Sastri VR (2014) Polymer additives used to enhance material properties for medical device applications. In: Sastri VR (ed) Plastics in medical devices: properties, requirements and applications. Elsevier, Amsterdam, pp 55–72
El-Sarraf MA, Abdo AE (2013) Insulating epoxy/barite and polyester/barite composites for radiation attenuation. Appl Radiat Isot 79:18–24
Kim SC, Dong KR, Chung WK (2012) Medical radiation shielding effect by composition of barium compounds. Ann Nucl Energy 47:1–5
Kobayashi S, Hosoda N, Takashima R (1997) Tungsten alloys as radiation protection materials. Nucl Instrum Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip 390:426–430. https://doi.org/10.1016/S0168-9002(97)00392-6
Noor NZA, Siddiqui SA, Hart R, Low IM (2013) Effect of particle size, filler loadings and X-ray tube voltage on the transmitted X-ray transmission in tungsten oxide—epoxy composites. Appl Radiat Isot 71:62–67. https://doi.org/10.1016/j.apradiso.2012.09.012
Auffan M, Rose J, Bottero J-Y et al (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nano 4:634–641
Warheit DB (2004) Nanoparticles. Mater Today 7:32–35. https://doi.org/10.1016/S1369-7021(04)00081-1
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976. https://doi.org/10.1016/j.envint.2006.06.014
Taurozzi JS, Hackley VA, Wiesner MR (2011) Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment—issues and recommendations. Nanotoxicology 5:711–729. https://doi.org/10.3109/17435390.2010.528846
Warheit DB, Sayes CM, Reed KL, Swain KA (2008) Health effects related to nanoparticle exposures: environmental, health and safety considerations for assessing hazards and risks. Pharmacol Ther 120:35–42. https://doi.org/10.1016/j.pharmthera.2008.07.001
Gould P (2004) Nanoparticles probe biosystems. Mater Today 7:36–43. https://doi.org/10.1016/S1369-7021(04)00082-3
Friedman H, Singh MS (2003) Radiation transmission measurements for Demron fabric. United States
El HF, Froyer G (2008) Transparent polymers embedding nanoparticles for X-rays attenuation. J Univ Chem Technol Metall 43:221
Gaier JR, Hardebeck WC, Bunch JRT et al (1998) Effect of intercalation in graphite epoxy composites on the shielding of high energy radiation. J Mater Res 13:2297–2301. https://doi.org/10.1557/JMR.1998.0320
Zhang W, Zeng J, Liu L, Fang Y (2004) A novel property of styrene-butadiene-styrene/clay nanocomposites: radiation resistance. J Mater Chem 14:209–213. https://doi.org/10.1039/B307978C
Azman NZN, Siddiqui SA, Hart R, Low IM (2013) Microstructural design of lead oxide-epoxy composites for radiation shielding purposes. J Appl Polym Sci 128:3213–3219. https://doi.org/10.1002/app.38515
Burgess IF (2009) The mode of action of dimeticone 4% lotion against head lice Pediculus capitis. BMC Pharmacol 9:3. https://doi.org/10.1186/1471-2210-9-3
Fujii T (2002) PDMS-based microfluidic devices for biomedical applications. Microelectron Eng 61:907–914. https://doi.org/10.1016/S0167-9317(02)00494-X
Sánchez-Arévalo FM, Garnica-Palafox IM, Jagdale P, Hernández-Cordero J, Rodil SE, Okonkwo AO, Robles Hernandez FC, Tagliaferro A (2015) Photomechanical response of composites based on PDMS and carbon soot nanoparticles under IR laser irradiation. Opt Mater Express 5:1792–1805
Duffy DC, McDonald JC, Schueller OJA, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984. https://doi.org/10.1021/ac980656z
Jagdale P, Castellino M, Marrec F et al (2014) Nano sized bismuth oxy chloride by metal organic chemical vapour deposition. Appl Surf Sci 303:250–254. https://doi.org/10.1016/j.apsusc.2014.02.158
Ortiz-Acosta D (2012) Historical material analysis of DC745U pressure pads. USA
Bird J (2015) Science for engineering, 5th edn. Taylor & Francis, Routledge
https://physics.nist.gov/PhysRefData/XrayMassCoef/ElemTab/z83.html
Mahdi KH, Ahmed ZS, Mkhaiber AF (2012) Calculation and study of gemma ray attenuation coefficients for different composites. J Pure Appl Sci 25:133–141
Anthony JW, Bideaux RA, Bladh KW, Nichols MC (eds) (2011) Bismoclite. In: Handbook of mineralogy. Mineralogical Society of America, Chantilly, VA
Masterton WL, Hurley CN (2008) Chemistry: principles and reactions. Cengage Learning, Boston, MA
Fricke BL, Donnelly LF, Frush DP et al (2003) In-plane bismuth breast shields for pediatric CT: effects on radiation dose and image quality using experimental and clinical data. Am J Roentgenol 180:407–411. https://doi.org/10.2214/ajr.180.2.1800407
Einstein AJ, Elliston CD, Groves DW et al (2012) Effect of bismuth breast shielding on radiation dose and image quality in coronary CT angiography. J Nucl Cardiol 19:100–108. https://doi.org/10.1007/s12350-011-9473-x
Cho JH, Kim MS, Rhim JD (2015) Comparison of radiation shielding ratios of nano-sized bismuth trioxide and molybdenum. Radiat Eff Defects Solids 170:651–658. https://doi.org/10.1080/10420150.2015.1080703
Hossain M, Su M (2012) Nanoparticle location and material-dependent dose enhancement in X-ray radiation therapy. J Phys Chem C 116:23047–23052. https://doi.org/10.1021/jp306543q
Krause W (1999) Delivery of diagnostic agents in computed tomography. Adv Drug Deliv Rev 37:159–173. https://doi.org/10.1016/S0169-409X(98)00105-7
Numburi UD, Schoenhagen P, Flamm SD et al (2010) Feasibility of dual-energy CT in the arterial phase: imaging after endovascular aortic repair. Am J Roentgenol 195:486–493. https://doi.org/10.2214/AJR.09.3872
Hoffmann R, Jansen C, König A et al (2001) Stent design related neointimal tissue proliferation in human coronary arteries; an intravascular ultrasound study. Eur Heart J 22:2007–2014
Butany J, Carmichael K, Leong SW, Collins MJ (2005) Coronary artery stents: identification and evaluation. J Clin Pathol 58:795–804. https://doi.org/10.1136/jcp.2004.024174
Acknowledgements
The author thanks and acknowledge Mr. Mauro Raimondo for FESEM analysis, Mr. Edgardo Berea Montes for providing the commercial grade Bismuth oxy chloride, and Dr. Marco Ganci for the support during the X-ray analysis of the composite samples. This work is financially support by European commission under PHOSCLEEN Project.
Funding
Funding was provided by FP7 Ideas: European Research Council (Grant Agreement No. 318977).
Author information
Authors and Affiliations
Contributions
PJ helped in concept, planning, preparation of nanomaterial, composite, and manuscript; MR contributed to data analysis, interpretation, and manuscript composition; RR was involved in carrying out measurements; CV helped in carrying out measurements; FB was involved in carrying out measurements; GC helped in carrying out measurements; AT was involved in conception, experimental design, and manuscript composition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jagdale, P., Rovere, M., Ronca, R. et al. Determination of the X-ray attenuation coefficient of bismuth oxychloride nanoplates in polydimethylsiloxane. J Mater Sci 55, 7095–7105 (2020). https://doi.org/10.1007/s10853-020-04498-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04498-6