Abstract
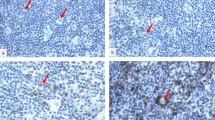
Acute or lymphomatous type adult T cell leukemia/lymphoma (ATLL) is an aggressive hematopoietic malignancy with poor prognosis. We previously reported that programmed cell death ligand 1 (PD-L1) expression could predict ATLL outcomes. However, the roles of other immune checkpoint molecules remain largely unknown in ATLL. Our aim in this study was to explore the clinicopathological impacts of immune checkpoint molecules in ATLL. Immunohistochemistry was performed in 69 ATLL patients with antibodies against the following: PD-L1, programmed cell death ligand 2 (PD-L2), OX40, OX40 ligand (OX40L), CD137, CD137 ligand (CD137L), Galectin-9, T cell immunoglobulin mucin-3 (Tim-3), cytotoxic T lymphocyte associated protein-4 (CTLA-4), lymphocyte activating-3 (LAG-3), CD80, CD86, glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR), GITR ligand (GITRL), and programmed death-1 (PD-1). Immune checkpoint molecules were variably expressed on neoplastic and/or microenvironmental cells. Expression of PD-1, OX40L, Galectin-9, and PD-L1 was nearly mutually exclusive on neoplastic cells, suggesting that immune checkpoint pathways differ in patients. Microenvironmental expression of PD-L1, OX40L, and Tim-3 was significantly associated with better overall survival (log-rank test; P =0.0004, 0.0394, and 0.0279, respectively). Univariate and multivariate analyses with clinical prognostic factors identified microenvironmental expression of PD-L1 and OX40L, and age (> 70 years) as significant prognostic factors. This is the first comprehensive analysis of ATLL immune checkpoint molecules. Our results may provide information on new therapeutic strategies in ATLL.




Similar content being viewed by others
References
Ohshima K, Jaffe E, Yoshino T, Siebert R (2017) Adult T-cell leukemia/lymphoma. In: Swerdlow SH, campo CE, Harris NL, et al., editors. World Health Organization classification of tumours. Revised 4th edn. IARC press, Lyon, pp 363–367
Shimoyama M (1991) Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia–lymphoma. A report from the lymphoma study group (1984–87). Br J Haematol 79:428–437. https://doi.org/10.1111/j.1365-2141.1991.tb08051.x
Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, Yokoyama S, Taniguchi H, Sasaki Y, Kurita D, Kawamoto K, Kato K, Imaizumi Y, Seto M, Ohshima K (2016) PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 128:1374–1381. https://doi.org/10.1182/blood-2016-02-698936
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Pennnock GK, Chow LQM (2015) The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist 20:812–822. https://doi.org/10.1634/theoncologist.2014-0422
Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A (2016) Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 52:50–66. https://doi.org/10.1016/j.ejca.2015.08.021
Croft M, So T, Soroosh P (2009) The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 229:173–191. https://doi.org/10.1111/j.1600-065x.2009.00766.x
Liu K, Ye H, Zhou J et al (2018) OX40 regulates the conversion and suppressive function of double-negative regulatory T-cells. Int Immunopharmacol 65:16–22. https://doi.org/10.1016/j.intimp.2018.09.035
Omar HA, Tolba MF (2019) Tackling molecular targets beyond PD-1/PD-L1: novel approaches to boost patient’s response to cancer immunotherapy. Crit Rev Oncol Hematol 135:21–29. https://doi.org/10.1016/j.critrevonc.2019.01.009
Chou FC, Chen HY, Kuo CC et al (2018) Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci 19:E430. https://doi.org/10.3390/ijms19020430
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 44:989–1004. https://doi.org/10.1016/j.immuni.2016.05.001
Anderson AC (2012) Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol 24:213–216. https://doi.org/10.1016/j.coi.2011.12.005
Das M, Zhu C, Kuchroo VK (2017) Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 276:97–111. https://doi.org/10.1111/imr.12520
Acknowledgments
The authors thank Mayumi Miura, Kanoko Miyazaki, and Chie Kuroki for their technical assistance. The language and format of this manuscript have been edited by Editage (https://www.editage.com).
Funding
This work was supported in part by Grants-in-Aid from the Japan Agency for Medical Research and Development (grant number 15ck0106015h0002) (MS) and the Japan Society for the Promotion of Science (KAKENHI) (grant JP26460446) (KO), (grant number JP17K17894) (MT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (include name of committee + reference number) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 381 kb)
ESM 2
(PDF 353 kb)
Kaplan–Meier plots depicting the survival differences between patients positive and negative for immune checkpoint molecule expression on microenvironmental cells. mi, microenvironmental; OS, overall survival; GITR, glucocorticoid-Induced tumor necrosis receptor-related protein; PD-1, programmed death-1; CTLA-4, cytotoxic T lymphocyte-associated antigen; PD-L2, programmed cell death ligand-2; LAG-3; lymphocyte-activating 3.
Kaplan–Meier plots depicting the survival differences between patients positive and negative for immune checkpoint molecule expression on neoplastic cells. n, neoplastic; OS, overall survival; PD-L1, programmed cell death ligand-1; PD-1, programmed death-1.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Miyoshi, H., Nakashima, K. et al. Comprehensive immunohistochemical analysis of immune checkpoint molecules in adult T cell leukemia/lymphoma. Ann Hematol 99, 1093–1098 (2020). https://doi.org/10.1007/s00277-020-03967-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-03967-x