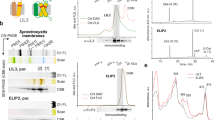
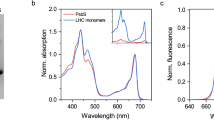
Abstract
Non-photochemical quenching is the photoprotective heat dissipation of chlorophyll-excited states. In higher plants, two quenching sites are located in trimeric LHCII and monomeric CP29 proteins. Catalysis of dissipative reactions requires interactions between chromophores, either carotenoid, chlorophyll or both. We identified CP29 protein domains involved in quenching by complementing an Arabidopsis deletion mutant with sequences deleted in pigment-binding or pH-sensitive sites. Acidic residues exposed to the thylakoid lumen were found not essential for activation of thermal dissipation in vivo. Chlorophylls a603 (a5) and a616 were identified as components of the catalytic pigment cluster responsible for quenching reaction(s), in addition to xanthophyll L2 and chlorophyll a609 (b5). We suggest that a conformational change induced by acidification in PsbS is transduced to CP29, thus bringing chlorophylls a603, a609 and a616 into close contact and activating a dissipative channel. Consistently, mutations on putative protonatable residues, exposed to the thylakoid lumen and previously suggested to regulate xanthophyll exchange at binding site L2, did not affect quenching efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession nos. At5g01530 (Lhcb4.1) and At3g08940 (Lhcb4.2). The knockout lines used in the work were obtained from the Nottingham Arabidopsis Stock Centre under stock nos. N376476 (koLhcb4.1) and N877954 (koLhcb4.2).
References
Nelson, N. & Ben Shem, A. The complex architecture of oxygenic photosynthesis. Nature 5, 1–12 (2004).
Van Amerongen, H. & Croce, R. Light-harvesting in Photosystem II. Photosynth. Res. 116, 251–263 (2013).
Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 56, 337–346 (2005).
Ruban, A. V., Johnson, M. P. & Duffy, C. D. The photoprotective molecular switch in the Photosystem II antenna. Biochim. Biophys. Acta 1817, 167–181 (2012).
Li, X. P. et al. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22866–22874 (2004).
Dominici, P. et al. Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J. Biol. Chem. 277, 22750–22758 (2002).
Wei, X. et al. Structure of spinach photosystem II-LHCII supercomplex at 3.2 A resolution. Nature 534, 69–74 (2016).
Betterle, N. et al. Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J. Biol. Chem. 284, 15255–15266 (2009).
Dall’Osto, L. et al. Two mechanisms for dissipation of excess light in monomeric and trimeric light-harvesting complexes. Nat. Plants 3, 17033 (2017).
de Bianchi, S., Dall’Osto, L., Tognon, G., Morosinotto, T. & Bassi, R. Minor antenna proteins CP24 and CP26 affect the interactions between Photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 20, 1012–1028 (2008).
de Bianchi, S. et al. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted Photosystem II macrostructure and are defective in photoprotection. Plant Cell 23, 2659–2679 (2011).
Pan, X. et al. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 18, 309–315 (2011).
Su, X. et al. Structure and assembly mechanism of plant C2S2M2-type PSII–LHCII supercomplex. Science 357, 815–820 (2017).
Ballottari, M., Dall’Osto, L., Morosinotto, T. & Bassi, R. Contrasting behavior of higher plant Photosystem I and II antenna systems during acclimation. J. Biol. Chem. 282, 8947–8958 (2007).
Walters, R. G., Ruban, A. V. & Horton, P. Identification of proton-active residues in a higher plant light- harvesting complex. Proc. Natl Acad. Sci. USA 93, 14204–14209 (1996).
Pesaresi, P., Sandona, D., Giuffra, E. & Bassi, R. A single point mutation (E166Q) prevents dicyclohexylcarbodiimide binding to the Photosystem II subunit CP29. FEBS Lett. 402, 151–156 (1997).
Bassi, R., Croce, R., Cugini, D. & Sandona, D. Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl Acad. Sci. USA 96, 10056–10061 (1999).
Caffarri, S., Kouril, R., Kereiche, S., Boekema, E. J. & Croce, R. Functional architecture of higher plant Photosystem II supercomplexes. EMBO J. 28, 3052–3063 (2009).
Croce, R., Cinque, G., Holzwarth, A. R. & Bassi, R. The soret absorption properties of carotenoids and chlorophylls in antenna complexes of higher plants. Photosynth. Res. 64, 221–231 (2000).
Cinque, G., Croce, R. & Bassi, R. Absorption spectra of chlorophyll a and b in Lhcb protein environment. Photosynth. Res. 64, 233–242 (2000).
Pietrzykowska, M. et al. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26, 3646–3660 (2014).
Fan, M. et al. Crystal structures of the PsbS protein essential for photoprotection in plants. Nat. Struct. Mol. Biol. 22, 729–735 (2015).
Sacharz, J., Giovagnetti, V., Ungerer, P., Mastroianni, G. & Ruban, A. V. The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat. Plants 3, 16225 (2017).
Pan, X., Liu, Z., Li, M. & Chang, W. Architecture and function of plant light-harvesting complexes II. Curr. Opin. Struct. Biol. 23, 515–525 (2013).
Ruban, A. V. et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578 (2007).
Duffy, C. D., Valkunas, L. & Ruban, A. V. Quantum mechanical calculations of xanthophyll–chlorophyll electronic coupling in the light-harvesting antenna of Photosystem II of higher plants. J. Phys. Chem. B 117, 7605–7614 (2013).
Ahn, T. K. et al. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320, 794–797 (2008).
Dall’Osto, L., Cazzaniga, S., North, H., Marion-Poll, A. & Bassi, R. The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photoxidative stress. Plant Cell 19, 1048–1064 (2007).
Dreuw, A., Fleming, G. R. & Head-Gordon, M. Chlorophyll fluorescence quenching by xanthophylls. Phys. Chem. Chem. Phys. 5, 3247–3256 (2003).
Niyogi, K. K., Grossman, A. R. & Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–1134 (1998).
Avenson, T. J. et al. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 283, 3550–3558 (2008).
Holt, N. E. et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 (2005).
Zhang, X., Henriques, R., Lin, S. S., Niu, Q. W. & Chua, N. H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646 (2006).
Casazza, A. P., Tarantino, D. & Soave, C. Preparation and functional characterization of thylakoids from Arabidopsis thaliana. Photosynth. Res. 68, 175–180 (2001).
Gilmore, A. M. & Yamamoto, H. Y. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 96, 635–643 (1991).
Schägger, H. & von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987).
Havaux, M., Dall’Osto, L., Cuine, S., Giuliano, G. & Bassi, R. The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J. Biol. Chem. 279, 13878–13888 (2004).
Baker, N. R. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113 (2008).
Acknowledgements
This work was supported by the HuntingLight grant from the University of Verona (to L.D.) and by EEC grant SE2B (no. 675006–SE2B) to R.B. We thank A. Zamboni (University of Verona) for help with statistical analysis, and S. Capaldi and S. Cazzaniga (University of Verona) for valuable advice and initial help with CP29 purification. We thank Z. Liu (Institute of Biophysics, Chinese Academy of Sciences, Beijing) for helpful discussions on CP29 structure.
Author information
Authors and Affiliations
Contributions
R.B. and L.D. conceived the work and designed the experiments. M.B. carried out the construction of mutants and performed their physiological characterization, together with L.D., Z.G. and R.C. Z.G. and R.C. analysed fluorescence quenching kinetics and biochemically characterized mutant lines. All authors contributed to writing the manuscript, discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Zhenfeng Liu and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–5, methods and references.
Rights and permissions
About this article
Cite this article
Guardini, Z., Bressan, M., Caferri, R. et al. Identification of a pigment cluster catalysing fast photoprotective quenching response in CP29. Nat. Plants 6, 303–313 (2020). https://doi.org/10.1038/s41477-020-0612-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0612-8
This article is cited by
-
The nature of carotenoid S* state and its role in the nonphotochemical quenching of plants
Nature Communications (2024)
-
Recent progress in atomistic modeling of light-harvesting complexes: a mini review
Photosynthesis Research (2023)
-
Generation and physiological characterization of genome-edited Nicotiana benthamiana plants containing zeaxanthin as the only leaf xanthophyll
Planta (2023)
-
Gene Expression, Hormone Signaling, and Nutrient Uptake in the Root Regermination of Grafted Watermelon Plants with Different Pumpkin Rootstocks
Journal of Plant Growth Regulation (2023)
-
A different perspective for nonphotochemical quenching in plant antenna complexes
Nature Communications (2021)