Abstract
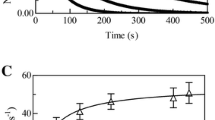
Haptoglobin (Hp) counterbalances the adverse effects of extra-erythrocytic hemoglobin (Hb) trapping the αβ dimers of Hb. In turn, the Hp:Hb complexes display heme-based reactivity. Here, the kinetics of cyanide and carbon monoxide dissociation from ferrous-ligated Hp:Hb complexes are reported at pH 7.0 and 20.0 °C. Cyanide dissociation from Hp1-1:Hb(II)-CN− and Hp2-2:Hb-CN− has been followed upon the dithionite-mediated conversion of ferric to ferrous-ligated Hp:Hb complexes. Values of kon for the dithionite-mediated reduction of Hp1-1:Hb(III)-CN− and Hp2-2:Hb(III)-CN− are (7.3 ± 1.1) × 106 M−1 s−1 and (6.2 ± 1.0) × 106 M−1 s−1, respectively. Values of the first-order rate constant (i.e., h) for cyanide dissociation from Hp1-1:Hb(II)-CN− and Hp2-2:Hb(II)-CN− are (1.2 ± 0.2) × 10−1 s−1 and (1.3 ± 0.2) × 10−1 s−1, respectively. CO dissociation from Hp:Hb(II)-CO complexes has been followed by replacing CO with NO. Values of the first-order rate constant (i.e., l) for CO dissociation from Hp1-1:Hb(II)-CO are (1.4 ± 0.2) × 10−2 s−1 and (6.2 ± 0.8) × 10−3 s−1, and those from Hp2-2:Hb(II)-CO are (1.3 ± 0.2) × 10−2 s−1 and (7.3 ± 0.9) × 10−3 s−1. Values of kon, h, and l correspond to those reported for the R-state of tetrameric Hb and isolated α and β chains. This highlights the view that the conformation of the Hb αβ-dimers bound to Hp1-1 and Hp2-2 matches that of the R-state of the Hb tetramer. Furthermore, unlike ferric Hb(III), ligated ferrous Hb(II) does not show an assembly-linked structural change.
Graphic abstract







Similar content being viewed by others
Abbreviations
- CCP domain:
-
Complement control protein domain
- CO:
-
Carbon monoxide
- Hb:
-
Human hemoglobin
- Hb(III):
-
Ferric Hb
- Hb(II):
-
Ferrous Hb
- Hp:
-
Human haptoglobin
- Hp1-1:
-
Phenotype 1-1 of Hp
- Hp1-1:Hb(III):
-
Ferric Hp1-1:Hb complex
- Hp1-1:Hb(II):
-
Ferrous Hp1-1:Hb complex
- Hp2-2:
-
Phenotype 2-2 of Hp
- Hp2-2:Hb(III):
-
Ferric Hp2-2:Hb complex
- Hp2-2:Hb(II):
-
Ferrous Hp2-2:Hb complex
- NO:
-
Nitrogen monoxide
- SP-like domain:
-
Serine protease-like domain
References
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law K, Moestrup SK (2001) Nature 409:198–201
Buehler PW, Abraham B, Vallelian F, Linnemayr C, Pereira CP, Cipollo JF, Jia Y, Mikolajczyk M, Boretti FS, Schoedon G, Alayash AI, Schaer DJ (2009) Blood 113:2578–2586
Kaempfer T, Duerst E, Gehrig P, Roschitzki B, Rutishauser D, Grossmann J, Schoedon G, Vallelian F, Schaer DJ (2011) J Proteome Res 10:2397–2408
Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW (2012) J Clin Invest 122:1444–1458
Alayash AI, Andersen CB, Moestrup SK, Bülow L (2013) Trends Biotechnol 31:2–3
Mollan TL, Jia Y, Banerjee S, Wu G, Kreulen RT, Tsai AL, Olson JS, Crumbliss AL, Alayash AI (2014) Free Radic Biol Med 69:265–277
Andersen CBF, Stødkilde K, Sæderup KL, Kuhlee A, Raunser S, Graversen JH, Moestrup SK (2017) Antioxid Redox Signal 26:814–831
Andersen CB, Torvund-Jensen M, Nielsen MJ, de Oliveira CL, Hersleth HP, Andersen NH, Pedersen JS, Andersen GR, Moestrup SK (2012) Nature 489:456–459
Kurosky A, Barnett DR, Lee TH, Touchstone B, Hay RE, Arnott MS, Bowman BH, Fitch WM (1980) Proc Natl Acad Sci USA 77:3388–3392
Wejman JC, Hovsepian D, Wall JS, Hainfeld JF, Greer J (1984) J Mol Biol 174:319–341
Wejman JC, Hovsepian D, Wall JS, Hainfeld JF, Greer J (1984) J Mol Biol 174:343–368
Polticelli F, Bocedi A, Minervini G, Ascenzi P (2008) FEBS J 275:5648–5656
Nagel RL, Gibson QH (1971) J Biol Chem 246:69–73
Stødkilde K, Torvund-Jensen M, Moestrup SK, Andersen CB (2014) Nat Commun 5:5487
Nagel RL, Wittenberg JB, Ranney HM (1965) Biochim Biophys Acta 100:286–289
Nagel RL, Gibson QH (1966) J Mol Biol 22:249–255
Brunori M, Alfsen A, Saggese U, Antonini E, Wyman J (1968) J Biol Chem 243:2950–2954
Gibson QH, Parkhurst LJ, Geraci G (1969) J Biol Chem 244:4668–4676
Alfsen A, Chiancone E, Antonini E, Waks M, Wyman J (1970) Biochim Biophys Acta 207:395–403
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. North Holland Publishing Co, Amsterdam, London
Chiancone E, Antonini E, Brunori M, Alfsen A, Lavialle F (1973) Biochem J 133:205–207
Perutz MF (1979) Annu Rev Biochem 48:327–386
Azarov I, He X, Jeffers A, Basu S, Ucer B, Hantgan RR, Levy A, Kim-Shapiro DB (2008) Nitric Oxide 18:296–302
Ascenzi P, Coletta M (2018) J Phys Chem B 122:11100–11107
Ascenzi P, De Simone G, Polticelli F, Gioia M, Coletta M (2018) J Biol Inorg Chem 23:437–445
Ascenzi P, Tundo GR, Coletta M (2018) J Inorg Biochem 187:116–122
Ascenzi P, Polticelli F, Coletta M (2019) Sci Rep 9:6780
White SL (1975) J Biol Chem 250:1263–1268
Ascenzi P, di Masi A, De Simone G, Gioia M, Coletta M (2019) J Biol Inorg Chem 24:247–255
Ascenzi P, De Simone G, Ciaccio C, Coletta M (2020) J Inorg Biochem 202:110814
Bellelli A, Antonini G, Brunori M, Springer BA, Sligar SG (1990) J Biol Chem 265:18898–18901
Brunori M, Antonini G, Castagnola M, Bellelli A (1992) J Biol Chem 267:2258–2263
Bolognesi M, Bordo D, Rizzi M, Tarricone C, Ascenzi P (1997) Prog Biophys Mol Biol 68:29–68
Milani M, Ouellet Y, Ouellet H, Guertin M, Boffi A, Antonini G, Bocedi A, Mattu M, Bolognesi M, Ascenzi P (2004) Biochemistry 43:5213–5221
Bolli A, Ciaccio C, Coletta M, Nardini M, Bolognesi M, Pesce A, Guertin M, Visca P, Ascenzi P (2008) FEBS J 275:633–645
Ascenzi P, Sbardella D, Santucci R, Coletta M (2016) J Biol Inorg Chem 21:511–522
Barbosa RM, Lopes Jesus AJ, Santos RM, Pereira CL, Marques CF, Rocha BS, Ferreira NR, Ledo A, Laranjin J (2011) Glob J Anal Chem 2:272–284
Franzini C, Cattozzo G, Besozzi M (1987) J Clin Chem Clin Biochem 25:183–184
Stitt F, Coryell CD (1939) J Am Chem Soc 61:1263–1266
Cox RP (1977) Biochem J 167:493–495
Bateman H (1910) Proc Camb Phyl Soc 15:423–427
Lambeth DO, Palmer G (1973) J Biol Chem 248:6095–6103
Olivas E, De Waal DJ, Wilkins RG (1977) J Biol Chem 252:4038–4042
Lister MW, Garvie RC (1959) Can J Chem 37:1567–1574
Balasubramanian S, Lambright DG, Simmons JH, Gill SJ, Boxer SG (1994) Biochemistry 33:8355–8360
Ascenzi P, Ciaccio C, Coletta M (2007) Biochem Biophys Res Commun 363:931–936
Cox RP, Hollaway MR (1977) Eur J Biochem 74:575–587
Antonini G, Bellelli A, Concetti A, Falcioni G, Brunori M (1994) Biochim Biophys Acta 1025:252–257
Antonini G, Bellelli A, Brunori M, Falcioni G (1996) Biochem J 314:533–540
Boffi A, Ilari A, Spagnuolo C, Chiancone E (1996) Biochemistry 35:8068–8074
Wireko FC, Abraham DJ (1992) Protein Eng 5:3–5
Gray HB, Winkler JR (1996) Annu Rev Biochem 65:537–561
Brunori M, Noble RW, Antonini E, Wyman J (1966) J Biol Chem 241:5238–5243
Unzai S, Eich R, Shibayama N, Olson JS, Morimoto H (1998) J Biol Chem 273:23150–23159
Gibson QH (1973) Proc Natl Acad Sci USA 70:1–4
Sharma VS, Vedvick TS, Magde D, Luth R, Friedman D, Schmidt MR, Ranney HM (1980) J Biol Chem 256:5879–5884
Lane-Serff H, MacGregor P, Peacock L, Macleod OJ, Kay C, Gibson W, Higgins MK, Carrington M (2016) Elife 5:e13044
Park SY, Yokoyama T, Shibayama N, Shiro Y, Tame JR (2006) J Mol Biol 360:690–701
Vandegriff KD, Le Tellier YC, Winslow RM, Rohlfs RJ, Olson JS (1991) J Biol Chem 266:17049–17059
Savino C, Miele AE, Draghi F, Johnson KA, Sciara G, Brunori M, Vallone B (2009) Biopolymers 91:1097–1107
Birukou I, Maillett DH, Birukova A, Olson JS (2011) Biochemistry 50:7361–7374
Jones EM, Monza E, Balakrishnan G, Blouin GC, Mak PJ, Zhu Q, Kincaid JR, Guallar V, Spiro TG (2014) J Am Chem Soc 136:10325–10339
Sawicki CA, Gibson QH (1977) J Biol Chem 252:7538–7547
Hydrogen cyanide, PubChem CID: 768
Perrella M, Davids N, Rossi-Bernardi L (1992) J Biol Chem 267:8744–8751
Kawamura K, Kagiyama S, Ogawa A, Yanase T (1972) Biochim Biophys Acta 285:22–27
Nakagawa A, Lui FE, Wassaf D, Yefidoff-Freedman R, Casalena D, Palmer MA, Meadows J, Mozzarelli A, Ronda L, Abdulmalik O, Bloch KD, Safo MK, Zapol WM (2014) ACS Chem Biol 9:2318–2325
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) J Comput Chem 25:1605–1612
Acknowledgements
This work was supported by the grant of Dipartimenti di Eccellenza, MIUR (Legge 232/2016, Articolo 1, Comma 314-337; to P.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ascenzi, P., De Simone, G., Tundo, G.R. et al. Kinetics of cyanide and carbon monoxide dissociation from ferrous human haptoglobin:hemoglobin(II) complexes. J Biol Inorg Chem 25, 351–360 (2020). https://doi.org/10.1007/s00775-020-01766-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01766-3