Abstract
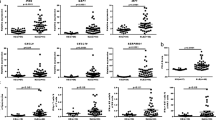
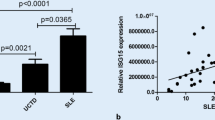
Gene expression analysis of peripheral blood cells may provide valuable information about the triggered molecular processes in systemic lupus erythematosus (SLE). The study aimed to quantify the mRNA in peripheral blood of seven target genes, including inflammatory cytokine genes (IL23A, IL12B, TNFA, IL18), and T regulatory-related genes (FOXP3, TGFB1, IL10) in patients with SLE and to correlate expression levels with disease activity and/or clinical manifestations. The relative quantification of target genes was performed using real-time polymerase chain reaction in peripheral blood obtained from 28 adult SLE females and 17 healthy women. The highest up-regulation in the blood of SLE patients was observed for IL23A with a median 9.54 (p < 0.0001), followed by TGFB1 (median: 2.07; p = 0.047) and IL10 (median: 1.84; p = 0.013). IL12B and TNFA were significantly down-regulated in patients compared to controls (median: 0.521; p = 0.0023, and median: 0.519; p = 0.0003, respectively). FOXP3 mRNA was lower among patients with higher degree of disease activity (median: 0.338; p = 0.029) and showed inverse correlation with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). IL18 mRNA correlated positively with the SLEDAI and was highly expressed during severe flares (median: 1.216; p = 0.021). IL18 up-regulation was associated with anti-dsDNA antibody positivity, while FOXP3 down-regulation with lupus nephritis. Our study pointed out the relationship of SLE disease activity and particular clinical manifestations with IL18 and FOXP3 expression, and the significant contribution of IL23A in the SLE immunopathogenesis. Hence, the peripheral blood cytokine mRNAs should be exploited as novel prognostic and diagnostic biomarkers.




Similar content being viewed by others
References
Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci 100:2610–2615. https://doi.org/10.1073/pnas.0337679100
Zhu H, Mi W, Luo H, Chen T, Liu S, Raman I, Zuo X, Li QZ (2016) Whole-genome transcription and DNA methylation analysis of peripheral blood mononuclear cells identified aberrant gene regulation pathways in systemic lupus erythematosus. Arthritis Res Ther 18:162. https://doi.org/10.1186/s13075-016-1050-x
Teruel M, Sawalha AH (2017) Epigenetic variability in systemic lupus erythematosus: what we learned from genome-wide DNA methylation studies. Curr Rheumatol Rep 19:32. https://doi.org/10.1007/s11926-017-0657-5
Petri M, Fu W, Ranger A, Allaire N, Cullen P, Magder LS, Zhang Y (2019) Association between changes in gene signatures expression and disease activity among patients with systemic lupus erythematosus. BMC Med Genomics 12:4. https://doi.org/10.1186/s12920-018-0468-1
Edwards CK 3rd, Green JS, Volk HD, Schiff M, Kotzin BL, Mitsuya H, Kawaguchi T, Sakata KM, Cheronis J, Trollinger D, Bankaitis-Davis D, Dinarello CA, Norris DA, Bevilacqua MP, Fujita M, Burmester GR (2012) Combined anti-tumor necrosis factor-α therapy and DMARD therapy in rheumatoid arthritispatients reduces inflammatory gene expression in whole blood compared to DMARD therapy alone. Front Immunol 3:366. https://doi.org/10.3389/fimmu.2012.00366
Larosa M, Zen M, Gatto M, Jesus D, Zanatta E, Iaccarino L, Inês L, Doria A (2019) IL-12 and IL-23/Th17 axis in systemic lupus erythematosus. Exp Biol Med 244:42–51. https://doi.org/10.1177/1535370218824547
Qiu F, Song L, Yang N, Li X (2013) Glucocorticoid downregulates expression of IL-12 family cytokines in systemic lupus erythematosus patients. Lupus 22:1011–1016. https://doi.org/10.1177/0961203313498799
Xia LP, Li BF, Shen H, Lu J (2015) Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand J Rheumatol 44:200–205. https://doi.org/10.3109/03009742.2014.962080
Fischer K, Przepiera-Będzak H, Sawicki M, Walecka A, Brzosko I, Brzosko M (2017) Serum interleukin-23 in polish patients with systemic lupus erythematosus: association with lupus nephritis, obesity, and peripheral vascular disease. Mediat Inflamm 2017:9401432. https://doi.org/10.1155/2017/9401432
Mende R, Vincent FB, Kandane-Rathnayake R, Koelmeyer R, Lin E, Chang J, Hoi AY, Morand EF, Harris J, Lang T (2018) Analysis of serum interleukin (IL)-1β and IL-18 in systemic lupus erythematosus. Front Immunol 9:1250. https://doi.org/10.3389/fimmu.2018.01250
Deuteraiou K, Kitas G, Garyfallos A, Dimitroulas T (2018) Novel insights into the role of inflammasomes in autoimmune and metabolic rheumatic diseases. Rheumatol Int 38:1345–1354. https://doi.org/10.1007/s00296-018-4074-5
Zhu Q, Kanneganti TD (2017) Cutting Edge: distinct regulatory mechanisms control proinflammatory cytokines IL-18 and IL-1β. J Immunol 198:4210–4215. https://doi.org/10.4049/jimmunol.1700352
Li W, Deng C, Yang H, Wang G (2019) The regulatory T cell in active systemic lupus erythematosus patients: a systemic review and meta-analysis. Front Immunol 10:159. https://doi.org/10.3389/fimmu.2019.00159
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6:345–352. https://doi.org/10.1038/ni1178
Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, Harris J (2016) Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep 6:34604. https://doi.org/10.1038/srep34604
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum 35:630–640
Abrahamowicz M, Fortin PR, du Berger R, Nayak V, Neville C, Liang MH (1998) The relationship between disease activity and expert physician’s decision to start major treatment in active systemic lupus erythematosus: a decision aid for development of entry criteria for clinical trials. J Rheumatol 25:277–284
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Miteva L, Goycheva MI, Manolova I, Vasilev G, Stoilov R, Stanilova S (2017) AB0005 Cytokine mRNAs gene expression associated with systemic lupus erythematosus. Ann Rheum Dis 76:1048–1048. https://doi.org/10.1136/annrheumdis-2017-eular.2299
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725. https://doi.org/10.1016/s1074-7613(00)00070-4
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748. https://doi.org/10.1038/nature01355
van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, Werth VP, Gordon RM, Zhou B, Hsu B, Chevrier M, Triebel M, Jordan JL, Rose S (2018) Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392:1330–1339. https://doi.org/10.1016/S0140-6736(18)32167-6
Du J, Li Z, Shi J, Bi L (2014) Associations between serum interleukin-23 levels and clinical characteristics in patients with systemic lupus erythematosus. J Int Med Res 42:1123–1130. https://doi.org/10.1177/0300060513509130
Wang X, Liu X, Zhang Y, Wang Z, Zhu G, Han G, Chen G, Hou C, Wang T, Ma N, Shen B, Li Y, Xiao H, Wang R (2016) Interleukin (IL)-39 [IL-23p19/Epstein–Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol 186:144–156. https://doi.org/10.1111/cei.12840
Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P (2014) CD4+ CD25highFOXP3+ T regulatory cells as a biomarker of disease activity in systemic lupus erythematosus: a prospective study. Clin Exp Rheumatol 32:630–639
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Nochy D, Debre P, Piette JC, Gorochov G (2005) Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 175:8392–8400. https://doi.org/10.4049/jimmunol.175.12.8392
Bai Y, Tong Y, Liu Y, Hu H (2018) Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin Exp Immunol 191:1–10. https://doi.org/10.1111/cei.13041
Italiani P, Manca ML, Angelotti F, Melillo D, Pratesi F, Puxeddu I, Boraschi D, Migliorini P (2018) IL-1 family cytokines and soluble receptors in systemic lupus erythematosus. Arthritis Res Ther 20:27. https://doi.org/10.1186/s13075-018-1525-z
Jafari-Nakhjavani MR, Abedi-Azar S, Nejati B (2016) Correlation of plasma interleukin-18 concentration and severity of renal involvement and disease activity in systemic lupus erythematosus. J Nephropathol 5:28–33. https://doi.org/10.15171/jnp.2016.05
El-Fetouh SA, Mohammed RHA, Abozaid HSM (2014) Serum interleukin-18 and interleukin-10 levels in systemic lupus erythematosus: correlation with SLEDAI score and disease activity parameters. Egypt Rheumatol Rehabil 41:160–166. https://doi.org/10.4103/1110-161X.147358
Pisetsky DS (2016) Anti-DNA antibodies-quintessential biomarkers of SLE. Nat Rev Rheumatol 12:102–110. https://doi.org/10.1038/nrrheum.2015.151
Mistry P, Kaplan MJ (2017) Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol 185:59–73. https://doi.org/10.1016/j.clim.2016.08.010
Jeremic I, Djuric O, Nikolic M, Vlajnic M, Nikolic A, Radojkovic D, Bonaci-Nikolic B (2019) Neutrophil extracellular traps-associated markers are elevated in patients with systemic lupus erythematosus. Rheumatol Int 39:1849–1857. https://doi.org/10.1007/s00296-019-04426-1
Magna M, Pisetsky DS (2015) The role of cell death in the pathogenesis of SLE: is pyroptosis the missing link? Scand J Immunol 82:218–224. https://doi.org/10.1111/sji.12335
Funding
This work was supported by Grants No. 2/2017 and No. 2/2018 from the Fund for Scientific and Mobile Project from the Trakia University, Medical Faculty, Stara Zagora, Bulgaria.
Author information
Authors and Affiliations
Contributions
LM, IM, MI, RS, and SS made substantial contributions to the conception or design of the work. MI and RS, assessed participants, analyzed and interpreted the data. LM, IM and SS performed the analyses, collected and interpreted the data. LM drafted the manuscript. RS and SS supervised the project. All authors read, revising and approved the final manuscript version and take full responsibility for the integrity of the study and the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Miteva L, Manolova I, Ivanova M, Stoilov R and Stanilova S declare that they have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local Ethics Committee of the University Hospital “St. Ivan Rilski”, Sofia, Bulgaria with a decision number #6, 29 Nov 2016.
Informed consent
Informed consent was obtained from all individual participants involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miteva, L.D., Manolova, I.M., Ivanova, M.G. et al. High interleukin-18 and low FOXP3 mRNAs in peripheral blood of women with severe systemic lupus erythematosus: a cross-sectional study. Rheumatol Int 40, 727–735 (2020). https://doi.org/10.1007/s00296-020-04542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04542-3