Abstract
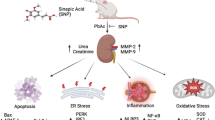
Lead (Pb) is one of the most common heavy metal pollutants affecting living organisms. It induces nephrotoxicity with significant alterations in renal structure and function. Luteolin (LUT) a flavonoid present in various plant products is well known for exhibiting numerous pharmacological properties. We evaluated the protective efficacy of LUT against Pb-induced renal injury in male Wistar rats. Four experimental groups: control, LUT (50 mg/kg, orally), PbAc (20 mg/kg, i.p.), LUT + PbAc (at the aforementioned doses) were maintained for 7 days. PbAc administration significantly increased renal Pb accumulation, urea, and creatinine levels in serum, and induced renal histological alterations. Additionally, compared to the control rats, PbAc-treated rats exhibited significantly low levels of antioxidant enzyme activity and expression (SOD, CAT, GPx and GR), as well as high MDA levels. Moreover, PbAc exposure downregulated Nfe212 and Homx1 mRNA expression and significantly increased inflammatory marker (TNF-α, IL-1β and NO) levels in renal tissue. PbAc significantly upregulated the synthesis of apoptotic related proteins and downregulated antiapoptotic protein expression. Notably, LUT pretreatment of PbAc-treated rats provided significant nephroprotection and reversed the alterations in the abovementioned parameters. In conclusion, LUT provided significant protection against PbAc intoxication via antioxidant, anti-inflammatory, and anti-apoptotic activities by activating the Nrf2/ARE signaling pathway.










Similar content being viewed by others
References
Abdel Moneim AE, Dkhil MA, Al-Quraishy S (2011) The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater 194:250–255
Wu X et al (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23(9):8244–8259
Baş H, Kalender Y (2016) Nephrotoxic effects of lead nitrate exposure in diabetic and nondiabetic rats: involvement of oxidative stress and the protective role of sodium selenite. Environ Toxicol 31(10):1229–1240
Dewanjee S et al (2013) Toxic effects of lead exposure in Wistar rats: involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves. Food Chem Toxicol 55:78–91
Liu B et al (2017) GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3β in rat kidney. Oncotarget 8(26):42226
Abdel-Moneim AM et al (2015) Curcumin ameliorates lead (Pb 2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 168(1):206–220
BaSalamah MA et al (2018) Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep 8(1):4853
Apaydın FG et al (2016) Subacute effects of low dose lead nitrate and mercury chloride exposure on kidney of rats. Environ Toxicol Pharmacol 41:219–224
Mabrouk A et al (2016) Protective effect of thymoquinone against lead-induced hepatic toxicity in rats. Environ Sci Pollut Res 23(12):12206–12215
Al Omar SY (2019) The neuroprotective role of coenzyme Q10 against lead acetate-induced neurotoxicity is mediated by antioxidant, anti-inflammatory and anti-apoptotic activities. Int J Environ Res Public Health 16(16):e2895
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 11(7):e0158965
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7(7):2745–2788
Ezejiofor AN, Orisakwe OE (2019) Nephroprotective effect of Costus afer on lead induced kidney damage in albino rats. International journal of physiology, pathophysiology and pharmacology 11(2):36
Al-Megrin WA et al (2019) Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants (Basel) 9(1):10
Al-Megrin WA et al (2020) Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front Physiol. https://doi.org/10.3389/fphys.2020.00064
Miceli N et al (2019) Phytochemical characterization and biological activities of a hydroalcoholic extract obtained from the aerial parts of Matthiola incana (L.) R. Br. subsp. incana (Brassicaceae) growing wild in Sicily (Italy). Chem Biodivers 16(4):e1800677
Soheili M, Salami M (2019) Lavandula angustifolia biological characteristics: an in vitro study. J Cell Physiol 234(9):16424–16430
Chen L et al (2016) Protective effect of luteolin on streptozotocin-induced diabetic renal damage in mice via the regulation of RIP140/NF-кB pathway and insulin signalling pathway. J Funct Foods 22:93–100
Xu N et al (2014) Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol Nutr Food Res 58(6):1258–1268
Baek K-S et al (2016) In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res 40(4):437–444
López-Lázaro M (2009) Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 9(1):31–59
Seelinger G, Merfort I, Schempp CM (2008) Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med 74(14):1667–1677
Akinrinde A, Adebiyi O (2019) Neuroprotection by luteolin and gallic acid against cobalt chloride-induced behavioural, morphological and neurochemical alterations in Wistar rats. Neurotoxicology 74:252–263
Alekhya Sita GJ et al (2019) Protective role of luteolin against bisphenol A‐induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/HO‐1 pathway. IUBMB life
Domitrović R et al (2013) Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 310:115–123
Arslan BY et al (2016) Luteolin ameliorates colistin-induced nephrotoxicity in the rat models. Renal Fail 38(10):1735–1740
Tan X et al (2018) Dietary luteolin protects against HgCl2-induced renal injury via activation of Nrf2-mediated signaling in rat. J Inorg Biochem 179:24–31
Hong X et al (2017) Luteolin treatment protects against renal ischemia-reperfusion injury in rats. Mediat Inflamm. https://doi.org/10.1155/2017/9783893
Xin S-B et al (2016) Protective effects of luteolin on lipopolysaccharide-induced acute renal injury in mice. Med Sci Monit 22:5173
Wang GG et al (2011) Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med 2011:323171
Kalbolandi SM et al (2019) Luteolin confers renoprotection against ischemia–reperfusion injury via involving Nrf2 pathway and regulating miR320. Mol Biol Rep 46:4039–4047
Moneim AEA (2012) Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res 148(3):363–370
Soliman MM, Baiomy AA, Yassin MH (2015) Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in wistar rats. Biol Trace Elem Res 167(1):91–102
Szkoda J, Zmudzki J (2005) Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bull Vet Inst Pulawy 49:89–92
Green LC et al (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Ferreiro CR et al (2001) Influence of hypoxia on nitric oxide synthase activity and gene expression in children with congenital heart disease: a novel pathophysiological adaptive mechanism. Circulation 103(18):2272–2276
El-Boshy ME et al (2019) The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ Sci Pollut Res 26(22):22736–22746
Liu C-M, Ma J-Q, Sun Y-Z (2010) Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ Toxicol Pharmacol 30(3):264–271
Al-Quraishy S et al (2016) Neuroprotective potential of Indigofera oblongifolia leaf methanolic extract against lead acetate-induced neurotoxicity. Neural Regen Res 11(11):1797–1803
Hasanein P, Teimuri-Far M (2015) Protective effect of bioactive peptide carnosine against lead-induced oxidative stress in kidney of rats. Cell Mol Biol (Noisy-le-Grand, France) 61(4):8–14
Kang KP et al (2010) Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol Dial Transplant 26(3):814–822
Mabrouk A (2019) Thymoquinone attenuates lead-induced nephropathy in rats. J Biochem Mol Toxicol 33(1):e22238
Domitrovic R et al (2009) Dose- and time-dependent effects of luteolin on carbon tetrachloride-induced hepatotoxicity in mice. Exp Toxicol Pathol 61(6):581–589
Zhang H et al (2017) Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-kappaB/P53 signaling pathway in rats. Oncotarget 8(25):40982–40993
Baiyun R et al (2018) Luteolin-mediated PI3K/AKT/Nrf2 signaling pathway ameliorates inorganic mercury-induced cardiac injury. Ecotoxicol Environ Saf 161:655–661
Jozefczak M et al (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13(3):3145–3175
Wang GG et al (2011) Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2011/323171.
Almaimani RA et al (2019) Enhanced remedial effects for vitamin D3 and calcium co-supplementation against pre-existing lead nephrotoxicity in mice: the roles of renal calcium homeostatic molecules. Biochim Biophys Acta (BBA) 1865(2):512–524
Metryka E et al (2018) Lead (Pb) exposure enhances expression of factors associated with inflammation. Int J Mol Sci 19(6):1813
Wang H et al (2016) Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol Cells 39(6):508
Yang D et al (2016) Regulation of Sirt1/Nrf2/TNF-alpha signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci Rep 6:37157
Narayana K, Al-Bader M (2011) Ultrastructural and DNA damaging effects of lead nitrate in the liver. Exp Toxicol Pathol 63(1):43–51
Bas H, Kalender S, Pandir D (2014) In vitro effects of quercetin on oxidative stress mediated in human erythrocytes by benzoic acid and citric acid. Folia Biol (Krakow) 62(1):59–66
Lakshmi BV, Sudhakar M, Aparna M (2013) Protective potential of Black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol 35(3):361–368
Zhang Y-C et al (2013) Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol 59:272–280
Myhrstad MC et al (2002) Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med 32(5):386–393
Heidari R et al (2019) The nephroprotective properties of taurine in colistin-treated mice is mediated through the regulation of mitochondrial function and mitigation of oxidative stress. Biomed Pharmacother 109:103–111
Yan Y et al (2019) Combination of metformin and luteolin synergistically protects carbon tetrachloride-induced hepatotoxicity: Mechanism involves antioxidant, anti-inflammatory, antiapoptotic, and Nrf2/HO-1 signaling pathway. Biofactors 45(4):598–606
Alekhya Sita GJ et al (2019) Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/HO-1 pathway. IUBMB Life 71(7):1041–1047
Wang ZK et al (2016) Alleviation of lead-induced apoptosis by puerarin via inhibiting mitochondrial permeability transition pore opening in primary cultures of rat proximal tubular cells. Biol Trace Elem Res 174(1):166–176
Liu G et al (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90(5):1193–1209
Kang KP et al (2011) Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol Dial Transplant 26(3):814–822
Chipuk JE et al (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303(5660):1010–1014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albarakati, A.J.A., Baty, R.S., Aljoudi, A.M. et al. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol Biol Rep 47, 2591–2603 (2020). https://doi.org/10.1007/s11033-020-05346-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05346-1