Abstract
Dissolved organic carbon (DOC) from Oa horizons has been proposed to be an important contributor for subsoil organic carbon stocks. We investigated the fate of DOC by directly injecting a DOC solution from 13C labelled litter into three soil depths at beech forest sites. Fate of injected DOC was quantified with deep drilling soil cores down to 2 m depth, 3 and 17 months after the injection. 27 ± 26% of the injected DOC was retained after 3 months and 17 ± 22% after 17 months. Retained DOC was to 70% found in the first 10 cm below the injection depth and on average higher in the topsoil than in the subsoil. After 17 months DOC in the topsoil was largely lost (− 19%) while DOC in the subsoil did not change much (− 4.4%). Data indicated a high stabilisation of injected DOC in the subsoils with no differences between the sites. Potential mineralisation as revealed by incubation experiments however, was not different between DOC injected in topsoil or subsoils underlining the importance of environmental factors in the subsoil for DOC stabilisation compared to topsoil. We conclude that stability of DOC in subsoil is primary driven by its spatial inaccessibility for microorganisms after matrix flow while site specific properties did not significantly affect stabilisation. Instead, a more fine-textured site promotes the vertical transport of DOC due to a higher abundance of preferential flow paths.
Similar content being viewed by others
Introduction
Subsoils have been recognised as an overlooked key component of the terrestrial carbon pool, containing between 27 and 77% of soil organic carbon (SOC) in mineral soils (Harrison et al. 2011; Rumpel and Kogel-Knabner 2011). Especially forest soils represent an important component of the global C cycle, due to their higher C stocks as compared to arable soils (Poeplau et al. 2011). Organic C in subsoils is characterised by generally high mean residence times and thus high mean apparent 14C ages (Rumpel et al. 2002; Voort et al. 2016; Wang et al. 1996). Beside roots, dissolved organic carbon (DOC) is a major source of fresh carbon (C) that enters subsoils (Kaiser and Guggenberger 2000). Nevertheless, quantitative data on the contribution and turnover of different compounds such as DOC entering subsoils are scarce (Kögel-Knabner 2017) The results from two synthesis papers showed that the input of DOC into forest subsoils is much higher than the output via leaching which means that a considerable portion of DOC is retained or mineralised in the subsoil (Kindler et al. 2011; Michalzik et al. 2001). Kalbitz and Kaiser (2008) estimated the contribution of DOC to the subsoil C stock of a Podzol to be in the range of 25–66% for the B and C horizon. Consequent questions are inter alia: what is the origin of this DOC, how does it reach subsoils and what drives its stability if it is stable at all?
In general there are different pathways how DOC can reach subsoil horizons. One way is the direct transport to subsoils via preferential flow paths (Hagedorn et al. 2015), which is particularly taking place at heavy rainfall events (Kaiser and Guggenberger 2005). Another possibility is the “continuous sorption and precipitation, combined with microbial processing and subsequent desorption and dissolution” as it was described by Kaiser and Kalbitz (2012) and is referred to as the cascade model. This model can explain higher 14C ages of organic C and of DOC in subsoils and has been confirmed in a laboratory flow experiment by Leinemann et al. (2018), where the mobilisation and replacement of mineral-associated organic matter by percolating DOC was quantified. Accordingly in a laboratory experiment Hagedorn et al. (2015) tested the importance of DOC from fresh litter along a soil chronosequence. They found that DOC was retained in the uppermost centimetres of the mineral soil, whereas non litter derived soil organic matter is leached. Conversely Rothstein et al. (2018) showed in a field experiment that the organic horizon and the subsoil of a Podzol are directly linked. In their study around 80% of the C entering the subsoil derived from the organic layer while the rest derived from DOC that was produced during the passage of water through the topsoil. Until now there are no field experiments, testing the effect of different substrates and textural differences on the transport of DOC in topsoils and subsoils. Since the saturated water conductivity strongly depends on the texture of a soil (Saxton and Rawls 2006) one should expect large differences in the DOC transport between a clayey and a sandy soil.
Furthermore, not the fresh litter as it was used by Hagedorn et al. (2015), but the humified organic layer (Oa horizon) is recognised as the main source for DOC reaching partly also deeper soil horizons (Qualls and Haines 1992; Rothstein et al. 2018; Schulze et al. 2011). It has been shown that DOC from Oa horizons has a higher stability than that from fresh litter, both in solution and associated with the mineral phase (Don and Kalbitz 2005; Kalbitz et al. 2005). This is due to the different composition of DOC released from the differently degraded forest floor horizons (Klotzbücher et al. 2013). Dissolved organic carbon from Oa horizons is characterised by a much greater aromaticity, complexity of molecules and smaller content of carbohydrates compared to DOC from fresh litter, leading to stronger sorption and higher intrinsic stability (Kalbitz et al. 2005). DOC from fresh beech litter for example, can be mineralised by 65% within 90 days while DOC from degraded and humified beech litter could be mineralised by 9.1% only within the same time in a liquid incubation experiment from Kalbitz et al. (2003). The amount of organic carbon (OC) that is dissolved from the different organic layers and in the mineral topsoil thereby depends on seasonal, pedological, vegetational and microbial characteristics (Don and Schulze 2008; Guggenberger et al. 1994; Kögel-Knabner 2002; Lee et al. 2018). Consequently it should behave in a different way compared to DOC from fresh litter during its passage through the soil. To the best of our knowledge there are no field experiments testing the behaviour of DOC derived from humified organic layers within a soil profile and also if different soil and environmental conditions play a role. But this would be important to know, since subsoils underlie different environmental conditions than topsoils which influence organic matter decomposability and stabilisation. This is, e.g., due to lower SOC contents (Don et al. 2013; Rumpel and Kogel-Knabner 2011), different microbial communities (Agnelli et al. 2004), different water and oxygen availabilities (Schneider et al. 2017), temperature dependent effects (Tückmantel et al. 2017), or the availability of fresh organic matter inputs (Fontaine et al. 2007). Most studies indicate, that rather physical protection mechanisms than inherent recalcitrance are responsible for long-term stabilisation of SOC (Marschner et al. 2008; Schöning and Kögel-Knabner 2006; von Lützow et al. 2008) and that SOC turnover is governed by microbial accessibility (Dungait et al. 2012). As DOC reaches the subsoil it gets sorbed to the mineral phase and is part of SOC. Thereby sorption of DOC in subsoils is related to the amount of clay sized particles like phyllosilicates (Barré et al. 2014) or iron- and aluminium (hydr)oxides (Kaiser and Zech 1996; Kindler et al. 2011). In acidic subsoils, especially poorly crystalline minerals have been considered to exert a large impact on organic matter stabilization (Mikutta et al. 2006). In a sorption experiment, Kaiser and Guggenberger (2000) have shown that on the other hand a high coverage of mineral surfaces with organic matter reduces the availability of these surfaces to adsorb DOC. Consequently, subsoils should be more capable for DOC sorption and stabilisation compared to topsoils and fine textured soils with higher capacities of free sorption sites should be more capable than coarse textured soils. A critical step to test these assumptions under field conditions is to detect the source of DOC and its fate in top- and subsoils, because in all parts of the soil DOC is produced by solubilisation of SOC or root litter and influenced by sorption/desorption processes, transport and microbial consumption. Even though DOC fluxes reaching the subsoil are small, their contribution to build up stabilised SOC in subsoils may be large (Hagedorn et al. 2012; Kalbitz et al. 2007). Isotopic labelling techniques are useful tools to follow the fate of DOC (Fröberg et al. 2009; Hagedorn et al. 2015; Kammer and Hagedorn 2011). Furthermore, laboratory experiments may be useful to identify distinct processes participating in the DOC turnover, but the combined effect of microbial turnover and flux conditions on the role and fate of DOC in subsoils can be only realistically quantified under field conditions. To the best of our knowledge, there is only the field study from Rothstein et al. (2018), that assesses the DOC contribution to subsoils. And this study is restricted to one soil type and the authors could not distinguish between roots and SOC from O horizons as source for DOC.
Thus, the goal of this study was to assess the stability of DOC in topsoils and subsoils under beech forest from different soil parent materials. Our approach was to inject 13C-labelled DOC from decomposed beech litter into topsoil, upper subsoil and deeper subsoil horizons of different beech forest sites. The soils include two Cambisols and a Luvisol. This allowed us to directly assess the stability of DOC under field conditions as it was proposed by Schmidt et al. (2011) and Campbell and Paustian (2015). The stability of indigenous SOC and of SOC derived from DOC sorption was assessed by a laboratory incubation experiment. We tested the hypotheses that (i) more injected DOC is retained in subsoils than in topsoils and in fine textured soils compared to coarse textured soils, due to higher capacity of free sorption sites, (ii) coarse textured soils facilitate a more homogeneous and deeper translocation of injected DOC than fine textured soils, and (iii) retained DOC is more stable in subsoils than in topsoils.
Material and methods
Site description
The field experiments were conducted at three sites under beech forest (Fagus sylvatica) with soils derived from different parent material (sand, red sandstone and loess). The soil at the first experimental site (near Nienburg (Weser), 51° 34′ 41.34″ N, 10° 3′ 54.6192 E) was classified as a Dystric Cambisol developed on Pleistocene fluvial and aoelian sandy deposits and will be referred to as “Sand” in the following. The mean annual temperature at this site is 9.7 °C and the annual precipitation amounts to 762 mm (Leinemann et al. 2016). The soil at the second site (near Ebergötzen, 51° 34′ 41.34″ N, 10° 3′ 54.6192 E) was a Dystric Cambisol, developed on Triassic upper red sandstone and therefore will be referred to as “Red Sandstone” in the following. Mean annual temperature and precipitation at this site are 8.3 °C and 794 mm respectively. The soil at the third site (near Rüdershausen, 51° 34′ 47.532″ N, 10° 14′ 33.8424 E) was a Luvisol developed on loess deposits. This site will be referred to as “Loess” and has a mean annual temperature of 8.5 °C and an average annual precipitation of 733 mm. For further details about the three sites see Kirfel et al. (2019). Selected soil properties are summarised in Table 1.
Injection experiment
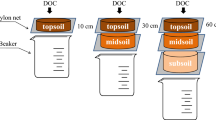
To trace the fate of DOC from decomposed litter in different soil layers, a 13C-labelled DOC solution derived from 13C-labelled beech litter was directly injected into three different depths increments at the three experimental sites from 7 May to 6 June 2017. We use the term DOC even though it is organic matter that comprises also other elements than carbon. The depths chosen for injection were 10, 50 and 100 cm. Due to the shallower soil development at the Red Sandstone, the injection there was set to 10, 30 and 60 cm depth. In the following, the depth increments are referred to as “Topsoil”, “Upper Subsoil” and “Deeper Subsoil”, respectively. To prepare the injection into the Upper and Deeper Subsoil, three pits per site were excavated down to 150 cm depth. These pits were located approximately 50 m apart from each other (Fig. 1). One horizontal shaft was cut into the profile wall for Upper Subsoil injection and one on the opposite site of the soil pit for the Deeper Subsoil injection. For the Topsoil injection, the upper 10 cm of the mineral soil was removed as an intact soil block, directly adjacent to the respective pit for the Upper and Deeper Subsoil. The DOC solution was produced by mixing decomposed 13C-labelled beech litter (δ13C of ~ 468 ‰) with de-ionised water in a 1:10 ratio for 12 h in a 250 l barrel with an electric stirrer. The decomposed beech litter consisted of a mixture of highly labelled beech litter (10 atom-%, IsoLife, Wageningen, The Netherlands) and unlabelled beech litter. This mixture was used in a field experiment for 22 months (Liebmann et al. 2020) before it was removed and used as DO13C source in this experiment. The obtained solution was pre-filtered to 2 mm via a tissue and finally filtered via cross-flow filtration (CMB 090, Microdyn-Nadir, Wuppertal, Germany) to < 0.2 µm. In total, 150 l of 13C-labelled DOC solution was produced. The DOC concentration of the solution was 200 mg l−1 with a δ13C value of 286 ‰. The DOC solution was injected with three field replicates (plots) per site. Additionally, one control plot per site was prepared with a 1 mmol CaCl2 solution being injected to test for disturbance and injection effects. Immediately before this solution was injected, one DOC sample per site was taken and frozen for further analyses. A qualitative analysis revealed a similar composition for all injected DOC samples. Further details are provided in the supplement (Table A1).
Injection of DOC at 10 cm depth at the Red Sandstone site (left) and in 50 and 100 cm depth at the Loess site (right). Note that on the right site, the injection at 100 cm depth is shown. The injection at 50 cm depth was conducted on the opposite site. The injection area was designed for three possible samplings. (Color figure online)
The injection was conducted with syringes which were filled with the DOC solution. Syringes were connected to 25 needles and combined at regular grid with on 20 × 20 cm plates. Plates were horizontally placed into the shafts and for the topsoil onto the excavated soil and the solution was slowly injected into the profile (Fig. 2). In total 1.8 l of the DOC solution was injected into an area of 400 cm2 corresponding to an added amount of 9 g DOC m−2 and thus resembling a precipitation event of 45 mm. Three plates with syringes were injected adjacent to each other per shaft to enable three samplings and to reduce side effects. Especially at the Loess, former root channels were detected at some the profile walls. During injection, parts of the injected DOC flowed out of some of these channels, indicating preferential flow. After injection, plates were removed and the shafts as well as the profiles were carefully filled with soil from the same depth and compacted to original bulk density. At the Topsoil injection spots the removed soil block was carefully returned to the same location where it came from. The respective injection areas were marked with iron bars on top of the restored soil profiles.
Concept of the sampling design for each of the three sites. The plots were approximately 50 m apart from each other. First sampling was conducted in August 2017, resulting in nine cores from the injection sites plus three respective control cores. Second sampling was conducted in October 2018. The water control (CaCl2) was completely sampled after 3 months
Sampling
In August 2017 and October 2018 (3 and 17 months after injection), three soil cores per site and injection depth, plus three adjacent control cores per site, were taken via a machine-driven percussion coring system (Nordmeyer Geotool, Berlin, Germany) (Fig. 1, plot 1–3). Additionally, three soil cores per site and injection depth from the CaCl2-control injection were sampled three months after injection (Fig. 1, plot 4). The amount of rainfall between injection and sampling after three months was as high as between 3 and 17 months after the injection due to a very dry summer period between the two sampling dates. In both periods of time precipitation amounted to ~ 300 mm at all three sites. Soil cores had a diameter of 6 cm and drilling depth was 100 cm below the respective injection depth, thus 200 cm deep for the Deep Subsoil injection. For the Red Sandstone, maximum drilling depth was 120 cm due to the shallow soil depth. Material above the injection depth was discarded. Material below the injection depth was separated into increments following defined depth Sects. (0–5, 5–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–80 and 80–100 cm below injection depth) resulting in 9 samples per core if possible and 934 samples in total for both samplings. For the Deeper Subsoil of the Red Sandstone, the deepest depth section consequently was 50–60 cm below injection depth. The respective control cores were separated into the same increments as the cores at the injection plots. Samples were filled into plastic bags and stored at 6 °C until further processing.
Chemical analyses and calculations
All soil samples were oven dried at 60 °C and sieved to 2 mm. Subsamples were homogenised, ground in a ball mill, and analysed for inorganic C, total C and nitrogen by dry combustion in an elemental analyser (LECO TruMac, St. Joseph, MI, USA). Organic C content was calculated by the difference between total and inorganic C. Carbonate was present in only very few samples and in very low concentrations (< 0.025 weight %). Respective values for bulk density, pH and stone content were obtained from a formerly conducted regional site grid sampling at the same sites (Heinze et al. 2018). Oxalate extractions were conducted according to Schwertmann (1964) and McKeague and Day (1966) by using a 0.2 M ammonium oxalate solution (pH 3) to dissolve poorly crystalline aluminosilicates and Fe hydroxides like ferrihydrite as well as organic complexes (Feo, Alo). Dithionite extractions were conducted according to (Mehra and Jackson 2013), modified by Sheldrick and McKeague (1975), to extract poorly crystalline as well as crystalline iron oxides (Fed, Ald).
Total SOC stocks (Mg ha−1) in each depth increment were calculated according Eq. 1,
where SOC is the soil organic carbon content in the fine soil < 2-mm fraction (mg g−1), BD is the bulk density of the fine soil (g cm−3), the stone content is the volume based proportion of stones (cm3 cm−3) and depth is the thickness of the depth increment (cm).
Homogenised samples were analysed for δ13C values in an isotope ratio mass spectrometer (Delta Plus, Thermo Fisher, Waltham, MA, USA) coupled to an elemental analyser (FLASH EA 1122 NA 1500; Wigan, United Kingdom). Because carbonate contents were so low and in the same range for a specific depth, we further measured δ13C without removing them to calculate the proportion of retained DOC. Resulting δ13C values (‰) were expressed relative to the international standard of Vienna Pee Dee Belemnite (V-PDB). δ13C values values from the labelled plots were compared with the upper quantile of a 90%-confidence interval from respective control samples calculated by Eq. 2:
Thereby the upper 90%-quantile (x(Q95)) is calculated by the mean (\(\stackrel{-}{x})\) and standard deviation (s) of the respective control samples from the same depth and both sampling dates (n = 6) and the value from the Student t-distribution·(tϕ;α). Only when the δ13C value of the labelled soil sample was higher than x(Q95), its value was taken into account for the calculation of a labelled DOC-derived SOC fraction. The fraction of labelled DOC-derived SOC in the bulk soil (f13C) was calculated with a two pool mixing model (Eq. 3) used by Cerri et al. (1985):
where δinject is the δ13C value (‰) of the labelled soil sample, δsolution is the δ13C value of the injected DOC solution and δcontrol is the mean δ13C value of the corresponding control samples.
With this fraction of labelled DOC-derived SOC the amount of retained DOC per depth increment (%) was calculated by Eq. 4:
where injected DOC is the amount of injected DOC in Mg ha−1. For both sampling dates, the amount of retained DOC per plot and injection depth was summed up over the whole sampling depth respectively. The final amount of retained DOC per injection depth and time was obtained by averaging values from the three plots.
Incubation experiment
To assess the potential stability of retained DOC against microbial decay a laboratory incubation experiment was conducted for 103 days at 20 °C. From each substrate, injection depth and plot we used three samples from within the top 20 cm below the respective injection depth (depth increments 0–5, 5–10 and 10–20 cm) taken from the sampling three months after injection. Samples were taken from the three plots per site plus respective samples from the same depth of the control soils. The samples were filled into 250 ml glass bottles (between 26 and 156 g for equivalent SOC ranges) and adjusted to 60% of their water holding capacity. As a control, four additional blank samples with burned quartz sand and four samples with ambient air were incubated, resulting in a total of 170 samples. Before starting the incubation, samples were pre-incubated for 1 week at 7 °C and for 2 weeks at 20 °C.
The potential C mineralisation was determined by measuring the CO2 production on five dates (after 1, 13, 27, 48, 103 days). At each sampling date incubation vessels were flushed with ambient air to reach a CO2 starting concentration near 400 ppm. Then, incubation vessels were closed gas-tight and four gas samples per soil sample and date were taken. The first two samples were taken directly after the bottles were closed. The lids contained a septum composed of a fluorelastomer material to keep them air-tight after sampling with a syringe needle. The other two gas samples were taken after a determined time interval (between 1 and 3 days) to ensure a sufficient accumulation of CO2. Samples were filled into evacuated gas vials (Labco Exetainer, Labco Limited, Lampeter, UK). One sample from the start and one sample after the time interval were analysed for CO2 concentrations by gas chromatography (Agilent 7890A, GC, Agilent Technologies, Santa Clara, USA) to account for the amount of accumulated CO2. The other two samples were analysed with an isotope ratio mass spectrometer (Delta Plus XP, Thermo Fisher Scientific, Bremen, Germany) to account for the development of δ13C of CO2 during the respiration, leading to a total amount of 3400 analysed gas samples.
The amount of respired CO2–C (mg CO2–C d−1) was calculated with Eq. 5.
where p is the pressure (mbar), xi is the difference of the CO2 concentration between the samplings (ppm), M is the molar mass of C (g mol−1), V the air volume of the sample (m3), R is the molar gas constant (J kmol−1 K−1), T is the temperature (K) and t is the elapsed time (d) between the samplings. This respiration rate was referred to the SOC content (called “SOC-normalised respiration”) by dividing it by the total amount of SOC in g in the sample. Since the content of inorganic carbon in the soil samples was extremely low we assumed that it has no considerable effect on the CO2 production (Bertrand et al. 2007).
To determine the amount of respired labelled material (called “labelled SOC-normalised respiration”) we also used the two pool mixing model (Eq. 2). For δcontrol we used median δ13C values of the respired CO2 from control samples from the three sites (Loess, Red Sandstone and Sand), injection depth (Top-, Upper and Deeper Subsoil), and sampling time (1, 13, 27, 48, 103) resulting in 9 observations per sampling date. The median was taken to reduce the influence of outliers on calculated labelled SOC-normalised respiration. In some cases only a small number of repetitions were obtained due to the fact that only samples from the labelled plots with significant amounts of retained DOC were taken into account. To account for natural fluctuations of the δ13CO2 values from the labelled samples we also included δ13CO2 values that showed more negative values than the control median.
Statistics
Statistical analyses were conducted using R Core Team (2018), including the packages glmmLasso (Groll and Tutz 2014) to perform generalised linear mixed effect analyses and ggplot2 (Wickham 2016) for graphical presentation. Significant differences of cumulative respiration normalised to SOC and labelled SOC after 103 days of incubation between the different sites and depths were tested with a Kruskal–Wallis test including a Wilcoxon posthoc analysis. The generalised linear mixed effect analysis was used to test for influencing parameters on the amount of retained DOC 3 months after injection. We only used the amount of retained DOC in the first depth increment below injection (0–5 cm). The mixed effect analysis was performed using SOC, AlO, FeO, FeD, substrate and horizon as fixed effects and the field replicates (plots) as a random effect. All numerical variables were standardized to a mean of 0 and a standard deviation of 1. Models were tested for deviations from homoscedasticity, normality of residuals and absence of collinearity. We did not allow for random slopes since we assumed that the effects of the included soil parameters were not variable across the plots. The fitted linear model did not have normally distributed residuals and were strongly heteroscedastic when we also included retained values of zero for modelling. Therefore we used only depth increments with significant amounts of retained DOC. This was also the reason why we could not perform a linear mixed effect analysis for the amount of retained DOC after 17 months, since the remaining samples did not contained enough data to provide reliable results.
Results
Amount of retained DOC
The average amounts of retained DOC in the first meter below injection after three months were 34 ± 11% for the Loess, 23 ± 9% for the Red Sandstone and 23 ± 7% for the Sand. Three months after injection more DOC was retained in the Topsoils (43 ± 35%) compared to the Subsoils (21 ± 17% in Upper Subsoils and 16 ± 14% in Deeper Subsoils) (Fig. 3). The amount of retained DOC accounted for only little OC in relation to the bulk SOC in the Topsoil (max. 0.5% of bulk SOC 3 months after injection) but quite high amounts in the Subsoils (max. 1.4% of bulk SOC in the Upper Subsoils and max. 4.8% of bulk SOC in the Deeper Subsoils) (Supplementary material, Fig. A2). For the Topsoil the maximum portion of 0.5% corresponds to 0.013 mg SOC g−1 soil. The highest value of 4.8% was obtained at the Deeper Subsoil of the Sand and corresponds to 0.053 mg SOC g−1 soil. Comparing the different sites, there was a decreasing trend of retained DOC from Loess to Red Sandstone and to Sand in Topsoils, whereas the retention in the Upper and Deeper Subsoil was similar for all sites. Due to the high within-group variability the differences between substrates and horizons were not statistical significant (Kruskal–Wallis rank sum test, p = 0.33).
The bulk SOC content was found to be the best predictor for the retained amounts of DOC after 3 months within the first 5 cm below injection depth increasing it by 3.2 ± 0.8% (p < 0.001) as revealed by the linear mixed effect model. Thus, topsoils retained more DOC than subsoils due to their higher SOC content. More DOC retention in SOC-rich soil was also found when Topsoils were excluded from the model, increasing the amount of retained DOC by 3.1 ± 0.9% (p < 0.001) per mg SOC g-1 soil. Thus, three months after injection more labelled DOC was retained in subsoils with high SOC contents compared to SOC poor subsoils.
Seventeen months after injection, the pattern of the retained labelled DOC changed. Highest amounts of retained DOC were found in the Deeper Subsoil of the Loess (41 ± 52%) and lowest in the Topsoil of the Sand (3 ± 5%). However, due to the small indigenous SOC contents, the retained amount after 17 months for the Deeper Subsoil of the Sand still represents 6.1 ± 10.6% of bulk SOC. Corresponding mean values averaged over all sites range from 11 ± 12% in Topsoils to 19 ± 20% in Upper Subsoils and 21 ± 32% in Deeper Subsoils. Thus, there was a change towards highest amounts of retained DOC in the Subsoils compared to the sampling after 3 months. The observed trends however, were not significant (Kruskal–Wallis rank sum test, p = 0.61). We partly found a higher amount of retained DOC after 17 months than after 3 months, especially for the Deeper Subsoil of the Loess, which we attribute to a high small-scale variability of the soils in terms of flow paths. The amount of retained DOC per plot reveals a high variation in the data (Table 2). Thus the average amounts of retained DOC per site and depth obtained extremely disparate values resulting in high standard deviations.
Translocation of DOC below the injection depths
We traced the 13C label within 100 cm below each injection depth to clarify as to which extent DOC was translocated downwards before adsorption to minerals, remobilisation from minerals or microbial immobilisation occurred. After 3 months, DOC injected into the Upper and Deeper Subsoils of all sites was largely restricted to the top 10 cm or even 5 cm below the injection depth (Fig. 4). In contrast, DOC injected into the Topsoil showed a comparatively deep translocation in particular at the Loess and Sand site. Especially the Topsoil of the Loess site showed a significant movement of DOC to 50—60 cm below injection.
Looking at the depth distribution 17 months after injection, the portion of DOC retained in the Topsoils decreased as compared to that after three months (Fig. 4). In the top 5 cm below the injection depth, no retained DOC was found any more after 17 months at the Loess and the Sand site. In the subsoils, we partly found a higher amount of retained DOC after 17 months than after three months, especially for the Upper Subsoil of the Loess with a strong translocation of injected DOC down to 70 cm below injection depth, which we attribute to a high small-scale variability of the soils in terms of flow paths (Fig. 4, lower panels). In general, there was only a decrease of retained DOC at the Red Sandstone and Sand suggesting no translocation but mainly decomposition.
Incubation results
After 103 days of laboratory incubation, in the Topsoil of the Loess, SOC-normalised respiration added up to 3.3 ± 0.6% within 103 days, which was significantly higher than respiration in the Upper and Deeper Subsoil with 1.2 ± 0.5 and 1.9 ± 0.7% (Fig. 5, Table 3). In contrast to the Loess, SOC-normalised respiration at the Sand and Red Sandstone showed no significant differences in potential respiration per SOC for different soil depths at all. Noteworthy, the more fine textured Loess did not show lower SOC-normalised respiration than the more sandy soils, and was even highest for the Topsoil. Averaged over all injection depths, the Sand showed the lowest respiration rates. When comparing the cumulative respiration normalized to bulk SOC with that from labelled SOC, eight out of nine samples tended to have higher values, except of the Topsoil samples from the Loess indicating a preferential respiration of labelled DOC than bulk SOC (Table 3). Due to the high variability of the labelled SOC-normalised respiration, these differences were not significant (p > 0.05, Kruskal–Wallis Test).
Cumulative bulk SOC-normalised (upper panel) and labelled SOC-normalised (lower panel) respiration in a 103 days laboratory incubation experiment. Values for SOC and labelled SOC-normalised respiration represent mean values from samples 0–5, 5–10 and 10–20 cm depth below injection with three repetitions per substrate (n = 9). Error bars represent standard errors. Different letters indicate significant differences (P < 0.05) for cumulative respiration after 103 days of incubation. Because only samples with positive recovery values were taken into account for labelled SOC-normalised respiration, number of observations strongly differs (loess: 5–6, red sandstone: 3–5, sand: 2–3). (Color figure online)
Behaviour of retained DOC in the field
The comparison of the amount of retained DOC after 17 months with that after three months allows assessing its stability under field conditions. Due to the depth distribution of retained DOC we exclude a translocation of retained DOC between 3 and 17 months to be responsible for the observed losses. Resulting losses, when existing, are therefore assigned to respiration processes. The loss of retained DOC in Topsoils was even higher between the two sampling dates (~ 19%) compared to the cumulative potential respiration during the incubation experiment, extrapolated to the same time period (~ 5%) (Table 3). This highlights the method dependency of estimated respiration rates and the importance of field studies to study DOC and SOC turnover. With regard to the amount of retained DOC at the Subsoils, four out of six field plots showed a lower loss compared to the incubation results. For the Deeper Subsoil of the Loess e.g. this was caused by a more pronounced retention of DOC after 17 months in greater depths, while the first 20 cm below injection showed a decreasing trend. We assume that this more pronounced retention after 17 months for the Deeper Subsoil of the Loess was due to a translocation directly after injection because of the increasing amounts with increasing depth (Fig. 4). Even though the estimation of field losses have to be handled with caution due to the mentioned problems, these values in general show the trend of more material being retained in the Upper and Deeper Subsoil (mean values of all samples: 19 ± 18 and 21 ± 31%) compared to the Topsoil (11 ± 12%). Additionally, a considerable amount of DOC being injected into the Topsoil was directly transferred to greater depths and thus became in fact part of the subsoil SOC (Fig. 4). The retained DOC within the top 40 cm below the topsoil injection at the Sand completely disappeared between 3 and 17 months after injection. Also the retained material at the first cm below injection at the topsoil injection at the Loess and Red Sandstone showed a strong decline.
Discussion
In contrast to laboratory experiments under controlled conditions, field experiments impose more challenges in terms of effort and data interpretation. This has also become evident in the results of our experiment. In four out of nine cases, mean retained material after 17 months was higher than after 3 months. This can be due to the observed lateral flow or because of an unequal distribution of injected DOC in the soil matrix. Nevertheless, due to the fact that a direct injection of DOC to topsoils and subsoils in the field was never done before, this study provides first information on the fate of DOC under real environmental conditions compared to laboratory incubation experiments.
Amount and distribution of injected DOC
Despite a partly deeper translocation, the amount of retained DOC in the first 10 cm below injection depth was comparatively high at the Topsoil increments for all sites after 3 months. This is contrary to our hypothesis that more DOC will be retained in Subsoils compared to Topsoils due to the higher availability of free sorption sites in the subsoil (Guggenberger and Kaiser 2003). According to Kaiser and Guggenberger (2000) a coverage of reactive mineral surfaces with organic matter should reduce the sorptive capacity. This was not the case for the Topsoils with their comparatively high amounts of SOC. Instead, the linear mixed effect model revealed that for the Topsoils, the SOC content was the best predictor for the amount of retained DOC after 3 months, confirming the findings of Vogel et al. (2014) in a mesocosm experiment. They have shown that new organic matter is preferentially attached to already present organo-mineral clusters. Injected DOC was therefore preferentially sorbed to already present organo-mineral clusters, while the amount of aluminium- or iron(hydr-)oxides was not crucial for the retention. These are partially unexpected field observations which stress the importance of studies in undisturbed soils, if possible under field conditions. Since these organo-mineral clusters represent microbial hotspots in the soil (Nannipieri et al. 2003), this would subsequently lead to a lower stabilisation of this retained DOC. This will be discussed in the stabilisation section later on.
Unlike expected, the more coarse sized Red Sandstone and Sand with their comparatively high water conductivity (Saxton and Rawls 2006) did not show a faster transport of the injected DOC compared to the fine textured Loess site. Furthermore, the Topsoil of the Loess and the Sand site showed a general deeper translocation of injected DOC after 3 months compared to the Subsoils. There are two explanations for the different translocations between the sites and the injection depths. One factor for a deeper distribution of injected DOC in Topsoils compared to Subsoils could be the decreasing water conductivity with increasing soil depth due to a higher bulk density (Table 1), leading to a longer contact time between DOC and the mineral phase and thus more efficient sorption and retention. The bulk density increased from 1.10 to 1.19 g cm−3 in the Topsoils to 1.32–1.54 g cm−3 in the Upper Subsoils. For a pure sand, Assouline (2006) has modelled a decrease of the saturated water conductivity from 236 mm h−1 at a bulk density of 1.25 g cm−3 to 112 mm h−1 at a bulk density of 1.48 g cm−3. Thus it can be assumed that the high water conductivity in the Topsoils led to a deeper infiltration of DOC before it was sorbed due to a short contact time Don and Schulze (2008). Besides that, the Topsoil of the Loess with its lower water conductivity even showed a deeper infiltration of injected DOC after three months. Therefore the other explanation that appears to be even more important for the depth distribution of injected DOC might be the abundance and stability of preferential flow paths. In general, macropores are recognised as the most important factor controlling preferential flow (Guo and Lin 2018). They are considered as possible pathways for DOC to reach deeper soil horizons (Don and Schulze 2008). Indications for more macropores at the Loess were the higher abundance of earthworm and root channels visible in soil profile. Such channels are less stable and abundant in more sandy soils such as at the Red Sandstone and Sand sites (Schneider and Don 2019). Nevertheless, to some extent preferential flow might be also important for the Sand and the Red Sandstone, since small amounts of retained DOC could also be found in greater depths. The deeper translocation of injected DOC at the Loess could therefore be a result of preferential flow directly after the injection. This is in agreement with the increasing amount of retained DOC with increasing depth in the deeper subsoil 17 months after injection (Fig. 4). A matrix flow between the sampling times seems unlikely since this would lead to a decreasing amount of retained material with increasing depth.
Despite this infiltration to greater depth due to preferential flow, the retention after three months was generally highest for all sites within the first centimetres below injection. This indicates a fast sorption even at the more sandy sites during the matrix flow. It is likely that there was enough time for the injected DOC to be retained within the first 10 cm below the injection depth, since even a structureless sandy soil can have very low infiltration rates as it was shown by Flury et al. (1994) with a dye infiltration experiment. This high retention potential of our investigated Sand is in agreement with studies from (Kalbitz et al. 2004; Nielsen et al. 1999) and was also apparent within field observations of Leinemann et al. (2016). The latter authors could observe a strong decline of transported DOC of 94% within 150 cm soil depth. Further it must be considered that injected DOC concentrations of 9 g m−2 present the upper limit of DOC concentrations that can reach forest subsoils within one year (Fröberg et al. 2007; Kindler et al. 2011; Rothstein et al. 2018). In our experiment this concentration was injected within hours. Nevertheless, injected DOC was retained within the first cm below injection depth. This indicates that the retention of DOC in subsoils is mainly due to the presence of free sorption sites (Kindler et al. 2011) which are by far not exhausted in our investigated soils independent of their texture.
Stability of topsoil and subsoil SOC
A bulk SOC-normalised respiration of 107 to 320 µg CO2–C g−1 C d−1 is within the range of comparable incubation experiments (Agnelli et al. 2004; Salome et al. 2010; Schrumpf et al. 2013; Soucemarianadin et al. 2018; Wordell-Dietrich et al. 2017). Significantly higher respiration rates for the Topsoil of the Loess compared to the Subsoil samples and a clear trend of higher SOC-normalised respiration at the Deeper Subsoil of the Sand compared to the Topsoil samples revealed relevant site dependent effects. Although confounding, these different courses for the Loess and the Sand can be explained by their substrate driven impacts on the mineralisation as follows: The higher SOC-normalised respiration of Deeper Subsoil compared to the Topsoil for the Sand in our incubation experiment could also be observed by Wordell-Dietrich et al. (2017) and Heitkötter et al. (2017), who conducted incubation experiments with soil from the same Sand site as in our experiment. A possible disturbance effect due to the destruction of stabilising aggregates (Salome et al. 2010) can be excluded here, since Vormstein (2017) also conducted incubation experiments with soil from the same site with undisturbed and sieved samples and could not find significant different respiration rates. An explanation could be a higher amount of particular organic matter (POM) compared to mineral associated organic matter (MOM) in the Deeper Subsoil of the Sand as a more easily degradable carbon source (Yakovchenko et al. 1998). This is indicated by a higher content of fine roots for the subsoil of the Sand site compared to the topsoil, shown by Kirfel et al. (2019) for samples from the same site. Despite this, stabilisation of SOC in acidic forest is mostly driven by poorly crystalline minerals (Kleber et al. 2015; Mikutta et al. 2006). This is due to their crystallinity, exhibiting a higher surface reactivity and thus a better protective capacity towards SOC (Mikutta et al. 2005). Hence, the higher amount of poorly crystalline minerals in the Upper Subsoil than in the Deeper Subsoil could be a factor for the differences between these both (Table 1). Interestingly, the comparatively high amounts of an easier degradable SOC source, indicated by a higher content of fine roots in the subsoil (Kirfel et al., 2019), and a lower content of stabilising poorly crystalline minerals did not lead to a higher SOC-normalised respiration of the Sand samples compared to the Loess and Red Sandstone. The Topsoil of the Loess with its highest SOC-normalised respiration even shows the highest amounts of SOC in the heavy fraction compared to the Topsoil of the Red Sandstone and the Sand (Vormstein, 2017). This could be a hint for the physical effect of textural differences on the SOC-normalised respiration. As it was shown by Preusser et al. (2019) for sand samples from the same site, a reduced bacterial utilisation of SOC is due to a spatial separation from C sources and low soil moisture in the highly sandy subsoil environment. Also the physiology of different microbial communities could be responsible for the different stability of SOC between the sites (Kallenbach et al. 2016). However, this was not investigated here.
Stability of injected DOC
The hypothesis of a higher stabilisation of injected DOC in Subsoils compared to Topsoils could be confirmed in our study, but with contrasting results regarding the incubation experiment and the loss between the two sampling dates. Our incubation results revealed no differences of potential labelled SOC-normalised respiration between Topsoil and Subsoils. A lack of significant differences between the labelled SOC-normalised respiration of Topsoil and Subsoil samples can be due to the high standard deviations of the incubations and the comparison of 9 groups which contained partly only two observations. Nevertheless, this was surprising, since the comparison of the amounts of DOC retained after 3 and 17 months revealed a strong decline in DOC retained in Topsoils and a stabilisation in Subsoils (Fig. 3). This may point to the fact that stabilisation effects in subsoils are largely driven by environmental factors that were not included in our incubation experiment, namely less temperature variation, a different moisture regime and an input of fresh bioavailable OC. The relatively strong decrease of injected DOC at the Topsoils after 17 months might be the result of a higher microbial activity, shown by the significantly higher respiration normalised to the bulk soil (Supplementary material, Fig. 4). Due to the fact that available energy sources in Topsoils are rather high compared to Subsoils, and injected DOC represents only a small amount of bulk SOC (Supplementary material, Fig. 1) it was likely less important as C and energy source for microorganisms in the Topsoils. In contrast, Subsoils are C poor and an addition of fresh organic substrate may immediately result in an increased microbial activity (Vogel et al. 2015) and higher mineralisation of added labile C (Kramer et al. 2013; Tian et al. 2016). Nevertheless, long-term stabilisation of SOC in Topsoils is rather hampered due to the probable accumulation of injected DOC to already present organo-mineral surfaces. This would explain the comparatively high amounts of retained DOC after 3 months in the Topsoils and the nearly complete mineralisation of this retained DOC within 14 months.
Contrary to our hypothesis, the more fine textured soils did not show higher amounts of stabilised DOC injected to the subsoils in the field. Per depth increment, there were no differences in the amounts of retained DOC after 17 months for the different sites. For the first cm below injection we can assume matrix flow conditions of injected DOC before it was sorbed to the mineral phase. Despite this, a considerable amount, especially at the Loess site, was translocated to greater depth via preferential flow paths before retention occurred. However, these rhizosphere habitats also represent the microbial hotspots in subsoil horizons (Bundt et al. 2001; Marschner et al. 2012), with higher specific enzyme activities compared to topsoil bulk SOC (Kramer et al. 2013). For the Sand site Wordell-Dietrich et al. (2019) showed that roots and root exudates in the Subsoil regions are the primary source of produced CO2. Injected DOC that was retained within these microbial hotspots might therefore be mineralised comparatively fast. In contrast, injected and retained DOC that has entered the soil via matrix flow might be spatial separated from these microbial hotspots and stabilised by poorly crystalline minerals. In conclusion the high stabilisation of injected DOC between the two sampling dates compared to the incubation results might be a result of its matrix flow and spatial separation from potential decomposers. The textural differences between the sites were rather responsible for a faster vertical movement, especially at the fine textured Loess, due to a higher abundance of preferential flow paths. Differences between the Upper and Deeper Subsoil were not substantial, pointing out to similar processes for subsoil OC stabilisation right from the spatial beginning of the subsoil.
Since retention of injected DOC with comparatively high concentrations occurred within the first 10 cm, it can be assumed that, for reaching deeper subsoils, this retained DOC requires permanent microbial degradation and translocation processes as described in the cascade model. Furthermore, as it was shown by Wordell-Dietrich et al. (in discussion), respired CO2 from subsoils primarily derives from roots and root exudates. Organic carbon bound to the mineral surfaces in the Subsoils of the bulk soil should therefore be relatively stable, not only because of the stabilising effect of organo-mineral complexes but also because it is not part of microbial hotspots.
Conclusion
Our results point out the importance of field experiments with regards to questions about the fate and stability of DOC in different soil depths and substrates. Due to the combination of stable isotope techniques with field experiments at multiple sites we were able to obtain two important findings: First, stabilisation of injected DOC in acidic forest subsoils might not be preferentially driven by the sorptive capacity, like the amount of poorly crystalline minerals of the soil, but by its spatial inaccessibility, i.e. the distance to microbial decomposers. Second, the direct transport of DOC from Oa layers to subsoils seems to be higher in fine-textured soils than in coarse textured soils due to their higher abundance of preferential flow paths. These flow paths also represent biological hotspots in subsoils. Thus, our results point to the importance of microbially-degraded and subsequently displaced OC through the soil matrix for the build-up of possibly stable SOC in subsoils. Unexpectedly, SOC itself facilitated the retention of DOC in the short term with more DOC retention in the topsoil than in the subsoil; but in the long-term DOC stabilisation on accessible mineral surfaces is required.
References
Agnelli A, Ascher J, Corti G, Ceccherini MT, Nannipieri P, Pietramellara G (2004) Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol Biochem 36(5):859–868
Assouline S (2006) Modeling the relationship between soil bulk density and the hydraulic conductivity function. Vadose Zone J 5(2):697–705
Barré P, Fernandez-Ugalde O, Virto I, Velde B, Chenu C (2014) Impact of phyllosilicate mineralogy on organic carbon stabilization in soils: incomplete knowledge and exciting prospects. Geoderma 235:382–395
Bertrand I, Delfosse O, Mary B (2007) Carbon and nitrogen mineralization in acidic, limed and calcareous agricultural soils: apparent and actual effects. Soil Biol Biochem 39(1):276–288
Bundt M, Widmer F, Pesaro M, Zeyer J, Blaser P (2001) Preferential flow paths: biological ‘hot spots’ in soils. Soil Biol Biochem 33(6):729–738
Campbell EE, Paustian K (2015) Current developments in soil organic matter modeling and the expansion of model applications: a review. Environ Res Lett 10(12):123004
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Austria
Don A, Kalbitz K (2005) Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol Biochem 37(12):2171–2179
Don A, Schulze ED (2008) Controls on fluxes and export of dissolved organic carbon in grasslands with contrasting soil types. Biogeochemistry 91(2–3):117–131
Don A, Rodenbeck C, Gleixner G (2013) Unexpected control of soil carbon turnover by soil carbon concentration. Environ Chem Lett 11(4):407–413
Dungait JA, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18(6):1781–1796
Flury M, Flühler H, Jury WA, Leuenberger J (1994) Susceptibility of soils to preferential flow of water: a field study. Water Resour Res 30(7):1945–1954
Fontaine S, Barot S, Barre P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450(7167):277–280
Fröberg M, Jardine PM, Hanson PJ, Swanston C, Todd D, Tarver J, Garten C (2007) Low dissolved organic carbon input from fresh litter to deep mineral soils. Soil Sci Soc Am J 71(2):347–354
Fröberg M, Hanson PJ, Trumbore SE, Swanston CW, Todd DE (2009) Flux of carbon from 14C-enriched leaf litter throughout a forest soil mesocosm. Geoderma 149(3–4):181–188
Groll A, Tutz G (2014) Variable selection for generalized linear mixed models by L 1-penalized estimation. Stat Comput 24(2):137–154
Guggenberger G, Kaiser K (2003) Dissolved organic matter in soil: challenging the paradigm of sorptive preservation. Geoderma 113(3–4):293–310
Guggenberger G, Zech W, Schulten H-R (1994) Formation and mobilization pathways of dissolved organic matter: evidence from chemical structural studies of organic matter fractions in acid forest floor solutions. Org Geochem 21(1):51–66
Guo L, Lin H (2018) Addressing two bottlenecks to advance the understanding of preferential flow in soils. Elsevier, Amsterdam, pp 61–117
Hagedorn F, Kammer A, Schmidt MW, Goodale CL (2012) Nitrogen addition alters mineralization dynamics of 13 C-depleted leaf and twig litter and reduces leaching of older DOC from mineral soil. Glob Change Biol 18(4):1412–1427
Hagedorn F, Bruderhofer N, Ferrari A, Niklaus PA (2015) Tracking litter-derived dissolved organic matter along a soil chronosequence using 14C imaging: biodegradation, physico-chemical retention or preferential flow? Soil Biol Biochem 88:333–343
Harrison RB, Footen PW, Strahm BD (2011) Deep soil horizons: contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. For Sci 57(1):67–76
Heinze S, Ludwig B, Piepho HP, Mikutta R, Don A, Wordell-Dietrich P, Helfrich M, Hertel D, Leuschner C, Kirfel K, Kandeler E, Preusser S, Guggenberger G, Leinemann T, Marschner B (2018) Factors controlling the variability of organic matter in the top- and subsoil of a sandy Dystric Cambisol under beech forest. Geoderma 311:37–44
Heitkötter J, Heinze S, Marschner B (2017) Relevance of substrate quality and nutrients for microbial C-turnover in top-and subsoil of a Dystric Cambisol. Geoderma 302:89–99
Kaiser K, Guggenberger G (2000) The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org Geochem 31(7–8):711–725
Kaiser K, Guggenberger G (2005) Storm flow flushing in a structured soil changes the composition of dissolved organic matter leached into the subsoil. Geoderma 127(3–4):177–187
Kaiser K, Kalbitz K (2012) Cycling downwards - dissolved organic matter in soils. Soil Biol Biochem 52:29–32
Kaiser K, Zech W (1996) Nitrate, sulfate, and biphosphate retention in acid forest soils affected by natural dissolved organic carbon. J Environ Qual 25(6):1325–1331
Kalbitz K, Kaiser K (2008) Contribution of dissolved organic matter to carbon storage in forest mineral soils. J Plant Nutr Soil Sci 171(1):52–60
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113(3–4):273–291
Kalbitz K, Glaser B, Bol R (2004) Clear-cutting of a Norway spruce stand: implications for controls on the dynamics of dissolved organic matter in the forest floor. Eur J Soil Sci 55(2):401–413
Kalbitz K, Schwesig D, Rethemeyer J, Matzner E (2005) Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol Biochem 37(7):1319–1331
Kalbitz K, Meyer A, Yang R, Gerstberger P (2007) Response of dissolved organic matter in the forest floor to long-term manipulation of litter and throughfall inputs. Biogeochemistry 86(3):301–318
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630
Kammer A, Hagedorn F (2011) Mineralisation, leaching and stabilisation of 13 C-labelled leaf and twig litter in a beech forest soil. Biogeosciences 8(8):2195–2208
Kindler R, Siemens J, Kaiser K, Walmsley DC, Bernhofer C, Buchmann N, Cellier P, Eugster W, Gleixner G, Grunwald T, Heim A, Ibrom A, Jones SK, Jones M, Klumpp K, Kutsch W, Larsen KS, Lehuger S, Loubet B, McKenzie R, Moors E, Osborne B, Pilegaard K, Rebmann C, Saunders M, Schmidt MWI, Schrumpf M, Seyfferth J, Skiba U, Soussana JF, Sutton MA, Tefs C, Vowinckel B, Zeeman MJ, Kaupenjohann M (2011) Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob Change Biol 17(2):1167–1185
Kirfel K, Heinze S, Hertel D, Leuschner C (2019) Effects of bedrock type and soil chemistry on the fine roots of European beech–a study on the belowground plasticity of trees. For Ecol Manag 444:256–268
Kleber M, Eusterhues K, Keiluweit M, Mikutta C, Mikutta R, Nico PS (2015) Mineral–organic associations: formation, properties, and relevance in soil environments. Advances in agronomy. Elsevier, Amsterdam, pp 1–140
Klotzbücher T, Kaiser K, Filley TR, Kalbitz K (2013) Processes controlling the production of aromatic water-soluble organic matter during litter decomposition. Soil Biol Biochem 67:133–139
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34(2):139–162
Kögel-Knabner I (2017) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter: 14 years on. Soil Biol Biochem 105:A3–A8
Kramer S, Marhan S, Haslwimmer H, Ruess L, Kandeler E (2013) Temporal variation in surface and subsoil abundance and function of the soil microbial community in an arable soil. Soil Biol Biochem 61:76–85
Lee MH, Park JH, Matzner E (2018) Sustained production of dissolved organic carbon and nitrogen in forest floors during continuous leaching. Geoderma 310:163–169
Leinemann T, Mikutta R, Kalbitz K, Schaarschmidt F, Guggenberger G (2016) Small scale variability of vertical water and dissolved organic matter fluxes in sandy Cambisol subsoils as revealed by segmented suction plates. Biogeochemistry 131(1–2):1–15
Leinemann T, Preusser S, Mikutta R, Kalbitz K, Cerli C, Hoschen C, Mueller CW, Kandeler E, Guggenberger G (2018) Multiple exchange processes on mineral surfaces control the transport of dissolved organic matter through soil profiles. Soil Biol Biochem 118:79–90
Liebmann P, Wordell-Dietrich P, Kalbitz K, Mikutta R, Kalks F, Don A, Woche SK, Dsilva LR, Guggenberger G (2020) Relevance of aboveground litter for soil organic matter formation—a soil profile perspective. Biogeosci Discuss 2020:1–29
Marschner B, Brodowski S, Dreves A, Gleixner G, Gude A, Grootes PM, Hamer U, Heim A, Jandl G, Ji R, Kaiser K, Kalbitz K, Kramer C, Leinweber P, Rethemeyer J, Schaeffer A, Schmidt MWI, Schwark L, Wiesenberg GLB (2008) How relevant is recalcitrance for the stabilization of organic matter in soils? J Plant Nutr Soil Sci 171(1):91–110
Marschner P, Marhan S, Kandeler E (2012) Microscale distribution and function of soil microorganisms in the interface between rhizosphere and detritusphere. Soil Biol Biochem 49:174–183
McKeague J, Day J (1966) Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can J Soil Sci 46(1):13–22
Mehra O, Jackson M (2013) Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. Clays and clay minerals. Elsevier, Amsterdam, pp 317–327
Michalzik B, Kalbitz K, Park J-H, Solinger S, Matzner E (2001) Fluxes and concentrations of dissolved organic carbon and nitrogen–a synthesis for temperate forests. Biogeochemistry 52(2):173–205
Mikutta R, Kleber M, Jahn R (2005) Poorly crystalline minerals protect organic carbon in clay subfractions from acid subsoil horizons. Geoderma 128(1–2):106–115
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77(1):25–56
Nannipieri P, Ascher J, Ceccherini M, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54(4):655–670
Nielsen KE, Ladekarl UL, Nørnberg P (1999) Dynamic soil processes on heathland due to changes in vegetation to oak and Sitka spruce. For Ecol Manag 114(1):107–116
Poeplau C, Don A, Vesterdal L, Leifeld J, Wesemael B, Schumacher J, Gensior A (2011) Temporal dynamics of soil organic carbon after land-use change in the temperate zone–carbon response functions as a model approach. Global Change Biol 17(7):2415–2427
Preusser S, Poll C, Marhan S, Angst G, Mueller CW, Bachmann J, Kandeler E (2019) Fungi and bacteria respond differently to changing environmental conditions within a soil profile. Soil Biol Biochem 137:107543
Qualls RG, Haines BL (1992) Biodegradability of dissolved organic matter in forest throughfall, soil solution, and stream water. Soil Sci Soc Am J 56(2):578–586
Rothstein DE, Toosi ER, Schaetzl RJ, Grandy AS (2018) Translocation of carbon from surface organic horizons to the subsoil in coarse-textured spodosols: implications for deep soil C dynamics. Soil Sci Soc Am J 82(4):969–982
Rumpel C, Kogel-Knabner I (2011) Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 338(1–2):143–158
Rumpel C, Kögel-Knabner I, Bruhn F (2002) Vertical distribution, age, and chemical composition of organic carbon in two forest soils of different pedogenesis. Org Geochem 33(10):1131–1142
Salome C, Nunan N, Pouteau V, Lerch TZ, Chenu C (2010) Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob Change Biol 16(1):416–426
Saxton KE, Rawls WJ (2006) Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci Soc Am J 70(5):1569–1578
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478(7367):49–56
Schneider F, Don A (2019) Root-restricting layers in German agricultural soils. Part II: adaptation and melioration strategies. Plant Soil 442(1–2):419–432
Schneider F, Don A, Hennings I, Schmittmann O, Seidel SJ (2017) The effect of deep tillage on crop yield—what do we really know? Soil Tillage Res 174:193–204
Schöning I, Kögel-Knabner I (2006) Chemical composition of young and old carbon pools throughout Cambisol and Luvisol profiles under forests. Soil Biol Biochem 38(8):2411–2424
Schrumpf M, Kaiser K, Guggenberger G, Persson T, Kögel-Knabner I, Schulze E-D (2013) Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10:1675–1691
Schulze K, Borken W, Matzner E (2011) Dynamics of dissolved organic 14 C in throughfall and soil solution of a Norway spruce forest. Biogeochemistry 106(3):461–473
Schwertmann U (1964) Differenzierung der eisenoxide des bodens durch extraktion mit ammoniumoxalat-lösung. Zeitschrift für Pflanzenernährung, Düngung, Bodenkunde 105(3):194–202
Sheldrick B, McKeague J (1975) A comparison of extractable Fe and Al data using methods followed in the USA and Canada. Can J Soil Sci 55(1):77–78
Soucemarianadin LN, Cecillon L, Guenet B, Chenu C, Baudin F, Nicolas M, Girardin C, Barre P (2018) Environmental factors controlling soil organic carbon stability in French forest soils. Plant Soil 426(1–2):267–286
Tian Q, Yang X, Wang X, Liao C, Li Q, Wang M, Wu Y, Liu F (2016) Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochemistry 128(1):125–139
Tückmantel T, Leuschner C, Preusser S, Kandeler E, Angst G, Mueller CW, Meier IC (2017) Root exudation patterns in a beech forest: dependence on soil depth, root morphology, and environment. Soil Biol Biochem 107:188–197
Vogel C, Mueller CW, Höschen C, Buegger F, Heister K, Schulz S, Schloter M, Kögel-Knabner I (2014) Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat Commun 5:2947
Vogel C, Heister K, Buegger F, Tanuwidjaja I, Haug S, Schloter M, Kögel-Knabner I (2015) Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions. Biol Fertil Soils 51(4):427–442
von Lützow M, Kögel-Knabner I, Ludwig B, Matzner E, Flessa H, Ekschmitt K, Guggenberger G, Marschner B, Kalbitz K (2008) Stabilization mechanisms of organic matter in four temperate soils: development and application of a conceptual model. J Plant Nutr Soil Sci 171(1):111–124
Voort TSVD, Hagedorn F, McIntyre C, Zell C, Walthert L, Schleppi P, Feng X, Eglinton TI (2016) Variability in 14 C contents of soil organic matter at the plot and regional scale across climatic and geologic gradients. Biogeosciences 13(11):3427–3439
Vormstein S (2017) Amount, composition and turnover of organic matter in topsoils and subsoils under mature beech forest. Faculty of Organic Agricultural Sciences University of Kassel, Witzenhausen, p 92
Wang Y, Amundson R, Trumbore S (1996) Radiocarbon dating of soil organic matter. Quatern Res 45(3):282–288
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York, p 259
Wordell-Dietrich P, Don A, Helfrich M (2017) Controlling factors for the stability of subsoil carbon in a Dystric Cambisol. Geoderma 304:40–48
Wordell-Dietrich P, Don A, Wotte A, Rethemeyer J, Bachmann J, Helfrich M, Kirfel K, Leuschner C (2019) Vertical partitioning of CO2 production in a Dystric Cambisol. Biogeosci Discuss 2019:1–27
Yakovchenko V, Sikora L, Millner P (1998) Carbon and nitrogen mineralization of added particulate and macroorganic matter. Soil Biol Biochem 30(14):2139–2146
Acknowledgement
Open Access funding provided by Projekt DEAL. This study was funded by the Deutsche Forschungsgemeinschaft (DFG) (DO1734/4–2) within the framework of the research unit SUBSOM (FOR1806)—“The Forgotten Part of Carbon Cycling: Organic Matter Storage and Turnover in Subsoils”. We would like to thank Frank Hegewald and the student assistants for their support in the field and in the laboratory, Roland Fuß for his detailed help regarding the application of a generalised linear mixed effect model and Jens Dyckmanns and Lars Szwec from the Centre for Stable Isotope Research and Analysis at the University of Göttingen for 13CO2 measurements. We would also like to thank the AK laboratory team for their support and patience with a disorder inducing experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Myrna Simpson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalks, F., Liebmann, P., Wordell-Dietrich, P. et al. Fate and stability of dissolved organic carbon in topsoils and subsoils under beech forests. Biogeochemistry 148, 111–128 (2020). https://doi.org/10.1007/s10533-020-00649-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00649-8