Abstract
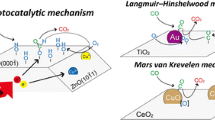
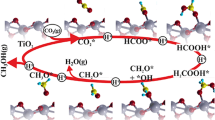
We report experimental and mechanistic understanding of methanol oxidation to produce methyl formate using CuO/TiO2-spindle composite as a promising photocatalyst under mild conditions with over 97% conversion and 83% selectivity. The catalysts are obtained via precise depositing of CuO nanoclusters (size: ~ 3.5 nm) at the {101} facet of the TiO2 to optimally tune exciton recombination through oxygen vacancies generation, evidenced by photoluminescence and Raman spectroscopy measurements. The turnover frequency (TOF) and the apparent quantum efficiency (AQE) of the 7%CuO/TiO2-spindle composites reach up to 23.8 molmethanol·gcat−1·h−1 and 55.2% at 25 °C, respectively, which are substantially higher than these previously reported photocatalysts. Further, the in-situ attenuated total reflection infrared spectroscopy analysis reveals that the methanol oxidation most likely takes place through the conversion of adsorbed methoxy (CH3O*) to formaldehyde (CHO*) intermediate, a subject of major debate for a long time. The adsorbed formaldehyde (CHO*) thus produced reacts with another CH3O* species in its close proximity to form the final product of methyl formate. Results of this study provide insights into the reaction mechanism, and offer guidelines to systematically develop and apply photocatalysts for methanol conversion and related reactions via surface engineering.

Similar content being viewed by others
References
Rong, L. Y.; Xu, Z. N.; Sun, J.; Guo, G. C. New methyl formate synthesis method: Coal to methyl formate. J. Energy Chem.2018, 27, 238–242.
Xu, B. J.; Liu, X. Y.; Haubrich, J.; Madix, R. J.; Friend, C. M. Selectivity control in gold-mediated esterification of methanol. Angew. Chem., Int. Ed.2009, 48, 4206–4209.
Meng, X. J.; Guo, H. Q.; Wang, Q.; Xiao, Y.; Chen, C. B.; Hou. B.; Li, D. B. Elucidating the nature and role of copper species in catalytic carbonylation of methanol to methyl acetate over copper/titania-silica mixed oxides. Catal. Sci. Technol.2017, 7, 3511–3523.
Kim, D. M.; Kim, A. R.; Chang, T. S.; Koo, H. M.; Kim, J. K.; Han, G. Y.; Shin, C. H.; Bae, J. W. Synergy effects of Al2O3 promoter on a highly ordered mesoporous heterogeneous Rh-g-C3N4 for a liquid-phase carbonylation of methanol. Appl. Catal. A: Gen.2019, 585, 117209.
Tatibouët, J. M. Methanol oxidation as a catalytic surface probe. Appl. Catal. A: Gen.1997, 148, 213–252.
He, L.; Liu, H. C.; Xiao C. X.; Kou, Y. Liquid-phase synthesis of methyl formate via heterogeneous carbonylation of methanol over a soluble copper nanocluster catalyst. Green Chem.2008, 10, 619–622.
Liu, H. C.; Iglesia, E. Selective oxidation of methanol and ethanol on supported ruthenium oxide clusters at low temperatures. J. Phys. Chem. B2005, 109, 2155–2163.
Forzatti, P.; Tronconi, E.; Elmi, A. S.; Busca, G. Methanol oxidation over vanadia-based catalysts. Appl. Catal. A: Gen.1997, 157, 387–408.
Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C. M.; Bäumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science2010, 327, 319–322.
Zhang, C. L.; Chen, Y. D.; Wang, H.; Li, Z. M.; Zheng, K.; Li S. J.; Li, G. Transition metal-mediated catalytic properties of gold nanoclusters in aerobic alcohol oxidation. Nano Res.2018, 11, 2139–2148.
Han, C. H.; Yang, X. Z.; Gao, G. J.; Wang, J.; Lu, H. L.; Liu, J.; Tong M.; Liang, X. Y. Selective oxidation of methanol to methyl formate on catalysts of Au-Ag alloy nanoparticles supported on titania under UV irradiation. Green Chem.2014, 16, 3603–3615.
Liang, X. Y.; Yang, X. Z.; Gao, G. J.; Li, C. F.; Li, Y. Y.; Zhang, W. D.; Chen, X. T.; Zhang, Y. B.; Zhang B. B.; Lei, Y. Q. et al. Performance and mechanism of CuO/CuZnAl hydrotalcites-ZnO for photocatalytic selective oxidation of gaseous methanol to methyl formate at ambient temperature. J. Catal.2016, 339, 68–76.
Liu, J.; Han, C. H.; Yang, X. Z.; Gao, G. J.; Shi, Q. Q.; Tong, M.; Liang X. Y.; Li, C. F. Methyl formate synthesis from methanol on titania supported copper catalyst under UV irradiation at ambient condition: Performance and mechanism. J. Catal.2016, 333, 162–170.
Shi, Q. Q.; Ping, G. C.; Wang, X. J.; Xu, H.; Li, J. M.; Cui, J. Q.; Abroshan, H.; Ding, H. J.; Li, G. CuO/TiO2 heterojunction composites: An efficient photocatalyst for selective oxidation of methanol to methyl formate. J. Mater. Chem. A2019, 7, 2253–2260.
Lichtenberger, J.; Lee, D.; Iglesia, E. Catalytic oxidation of methanol on Pd metal and oxide clusters at near-ambient temperatures. Phys. Chem. Chem. Phys.2007, 9, 4902–4906.
Li, N.; Wang, S. B.; Ren, Q. H.; Li, S. G.; Sun, Y. H. Catalytic mechanisms of methanol oxidation to methyl formate on vanadia-titania and vanadia-titania-sulfate catalysts. J. Phys. Chem. C2016, 120, 29290–29301
Ng, J.; Xu, S. P.; Zhang, X. W.; Yang, H. Y.; Sun, D. D. Hybridized nanowires and cubes: A novel architecture of a heterojunctioned TiO2/SrTiO3 thin film for efficient water splitting. Adv. Funct. Mater.2010, 20, 4287–4294.
Shi, Q. Q.; Li, Y.; Zhou, Y.; Miao, S.; Ta, N.; Zhan, E. S.; Liu, J. Y.; Shen, W. J. The shape effect of TiO2 in VOx/TiO2 catalysts for selective reduction of NO by NH3. J. Mater. Chem. A2015, 3, 14409–14415.
Tan, X. N.; Zhang, J. L.; Tan, D. X.; Shi, J. B.; Cheng, X. Y.; Zhang, F. Y.; Liu, L. F.; Zhang, B. X.; Su, Z. Z.; Han, B. X. Ionic liquids produce heteroatom-doped Pt/TiO2 nanocrystals for efficient photocatalytic hydrogen production. Nano Res.2019, 12, 1967–1972.
Zeng, Y. Q.; Wang, T. X.; Zhang, S. L.; Wang, Y. N.; Zhong, Q. Sol-gel synthesis of CuO-TiO2 catalyst with high dispersion CuO species for selective catalytic oxidation of NO. Appl. Surf. Sci.2017, 411, 227–234.
Meng, A. Y.; Zhang, J.; Xu, D. F.; Cheng, B.; Yu, J. G. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B2016, 198, 286–294.
Shao, B.; Zhao, W. N.; Miao, S.; Huang, J. H.; Wang, L. L.; Li, G.; Shen, W. J. Facet-dependent anchoring of gold nanoparticles on TiO2 for CO oxidation. Chin. J. Catal.2019, 40, 1534–1539.
Kong, J. J.; Rui, Z. B.; Liu, S. H.; Liu, H. W.; Ji, H. B. Homeostasis in CuxO/SrTiO3 hybrid allows highly active and stable visible light photocatalytic performance. Chem. Commun.2017, 53, 12329–12332.
Guo, S.; Zhang, S. H.; Fang, Q. H.; Abroshan, H.; Kim, H. J.; Haruta, M.; Li, G. Gold-palladium nanoalloys supported by graphene oxide and lamellar TiO2 for direct synthesis of hydrogen peroxide. ACS Appl. Mater. Interfaces2018, 10, 40599–40607.
Qamar, M. T.; Aslam, M.; Ismail I. M. I.; Salah, N.; Hameed, A. Synthesis, characterization, and sunlight mediated photocatalytic activity of CuO Coated ZnO for the removal of nitrophenols. ACS Appl. Mater. Interfaces2015, 7, 8757–8769.
Wang, X. F.; Li, T. Y.; Yu, R.; Yu, H. G.; Yu, J. G. Highly efficient TiO2 single-crystal photocatalyst with spatially separated Ag and F− bi-cocatalysts: Orientation transfer of photogenerated charges and their rapid interfacial reaction. J. Mater. Chem. A2016, 4, 8682–8689.
Yu, J. G.; Low, J.; Xiao, W.; Zhou P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc.2014, 136, 8839–8842.
Golestanbagh, M.; Parvini, M.; Pendashteh, A. Preparation, characterization and photocatalytic properties of visible-light-driven CuO/SnO2/TiO2 photocatalyst. Catal. Lett.2018, 148, 2162–2178.
Wu, Y.; Wei, Z. X.; Xu, R.; Gong, Y.; Gu, L.; Ma, J. M.; Yu, Y. Boosting the rate capability of multichannel porous TiO2 nanofibers with well-dispersed Cu nanodots and Cu2+-doping derived oxygen vacancies for sodium-ion batteries. Nano Res.2019, 12, 2211–2217.
Li, G. H; Dimitrijevic, N. M.; Chen, L.; Rajh T.; Gray, K. A. Role of surface/interfacial Cu2+ sites in the photocatalytic activity of coupled CuO-TiO2 nanocomposites. J. Phys. Chem. C2008, 112, 19040–19044.
Gervasini, A.; Manzoli, M.; Martra, G.; Ponti, A.; Ravasio, N.; Sordelli L.; Zaccheria, F. Dependence of copper species on the nature of the support for dispersed CuO catalysts. J. Phys. Chem. B2006, 110, 7851–7861.
Topalian, Z.; Stefanov, B. I.; Granqvist C. G.; Österlund, L. Adsorption and photo-oxidation of acetaldehyde on TiO2 and sulfate-modified TiO2: Studies by in situ FTIR spectroscopy and micro-kinetic modeling. J. Catal.2013, 307, 265–274.
Chiarello, G. L.; Ferri, D.; Selli, E. In situ attenuated total reflection infrared spectroscopy study of the photocatalytic steam reforming of methanol on Pt/TiO2. Appl. Surf. Sci.2018, 450, 146–154.
Wang, J.; Kispersky, V. F.; Delgass, W. N.; Ribeiro, F. H. Determination of the Au active site and surface active species via operando transmission FTIR and isotopic transient experiments on 2.3 wt.% Au/TiO2 for the WGS reaction. J. Catal.2012, 289, 171–178.
Engeldinger, J.; Domke, C.; Richter, M.; Bentrup, U. Elucidating the role of Cu species in the oxidative carbonylation of methanol to dimethyl carbonate on CuY: An in situ spectroscopic and catalytic study. Appl. Catal. A: Gen.2010, 382, 303–311.
Sun, Q.; Fu, Y. C.; Yang, H. X.; Auroux, A.; Shen, J. Y. Dehydration of methanol to dimethyl ether over Nb2O5 and NbOPO4 catalysts: Microcalorimetric and FT-IR studies. J. Mol. Catal. A: Chem.2007, 275, 183–193.
Kaminski, P. The application of FTIR in situ spectroscopy combined with methanol adsorption to the study of mesoporous sieve SBA-15 with cerium-zirconium oxides modified with gold and copper species. Arab. J. Chem.2020, 13, 851–862.
Acknowledgements
Q. Q. S. thanks the Postdoctoral Science Foundation of China (No. 223232), the Natural Science Foundation of Inner Mongolia Autonomous Region (No. 2018BS02004), the major special topics of Inner Mongolia science and technology department (No. 20181351), Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (No. NJYT-20-B20), the Program of Higher-level Talents of Inner Mongolia Agricultural University (No. NDYB2016-03), LiaoNing Revitalization Talents Program (No. XLYC1807121) and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2020_2719_MOESM1_ESM.pdf
Experimental and mechanistic understanding of photo-oxidation of methanol catalyzed by CuO/TiO2-spindle nanocomposite: Oxygen vacancy engineering
Rights and permissions
About this article
Cite this article
Shi, Q., Qin, Z., Yu, C. et al. Experimental and mechanistic understanding of photo-oxidation of methanol catalyzed by CuO/TiO2-spindle nanocomposite: Oxygen vacancy engineering. Nano Res. 13, 939–946 (2020). https://doi.org/10.1007/s12274-020-2719-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2719-7