Abstract
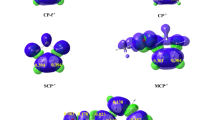
Density functional theory calculation is used to investigate the oxidation of cyclo-olefin (cyclobutene, cyclopentene, cyclohexene, cycloheptene, and cyclo-octene) by the complex [FeIV(O)(TQA)(NCMe)]2+, which has S = 2 ground state, and the effect of electronic factors and steric hindrance on reaction barriers. Our results suggest that the oxo–iron(IV) complex can oxidise C–H and C = C bonds via a single-state mechanism, and two different ways of electron transport exist. The energy barriers initially decrease with increasing substrate size, and the trend then reverses. Comparison of the energy barrier in different systems reveals that except for the reaction between [FeIV(O)(TQA)(NCMe)]2+ and cycloheptene, oxo–iron(IV) complexes prefer epoxidation to hydroxylation. However, the hydroxylated product is more stable than the corresponding epoxidated product. This result indicates that the products of epoxidation tend to decompose first. The energy barrier of hydroxylation and epoxidation originates from the balance of orbital interaction and Pauli repulsion from the equatorial ligand and protons on the approaching substrate. In this regard, we calculate the weak interaction between two fragments (oxo–iron complex and substrates) using the independent gradient model and drawn the corresponding 3D isosurface representations of reactants.










Similar content being viewed by others
References
Schwarz H (2011) Angew Chem Int Ed 50:10096–10115
Roudesly F, Oble J, Poli G (2017) J Mol Catal A: Chem 426:275–296
Huang XY, Groves JT (2018) Chem Rev 118:2491–2553
Mayank P, Que L Jr (2015) J Am Chem Soc 48:2443–2452
Solomon EI, Stahl SS (2019) Chem Rev 118:2299–2301
Schwarz H, Gonzalez-Navarrete P, Li JL, Schlangen M, Sun XY, Weiske T, Zhou SD (2017) Organometallics 36:8–17
Kirkland JK, Khan SN, Casale B, Miliordos E, Vogiatzis KD (2018) Phys Chem Chem Phys 20:28786–32879
Price JC, Barr EW, Tirupati B, Bollinger JM Jr, Krebs C (2003) Biochemistry 42:7497–7508
Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM Jr (2003) J Am Chem Soc 125:13008–13013
Grzyska PK, Hausinger RP, Proshlyakov DA (2010) Anal Biochem 399:64–71
Galonic DP, Barr EW, Walsh CT, Bollinger JM Jr, Krebs C (2007) Nat Chem Biol 3:113–116
Fujimori DG, Barr EW, Matthews ML, Koch GM, Yonce JR, Walsh CT, Bollinger JM Jr, Krebs C, Riggs-Gelasco PJ (2007) J Am Chem Soc 129:13408–13409
Panay AJ, Lee M, Krebs C, Bollinger JM Jr, Fitzpatrick PF (2011) Biochemistry 50:1928–1933
Matthews ML, Krest CM, Barr EW, Vaillancourt FH, Walsh CT, Green MT, Krebs C, Bollinger JM Jr (2009) Biochemistry 48:4331–4343
Shaun DW, Martin S, Megan LM, Lei VL, Yeonju K, Kiyoung P, Bell III CB, Ercan A, Jiyong Z, Yoshitaka Y, Shinji K, Makoto S, Carsten K, Martin JB, Edward IS (2013) Nature 499:320–323
Hoffart LM, Barr EW, Guyer RB, Bollinger JM Jr (2006) Krebs C. Proc Natl Acad Sci U S A 103:14738–14743
Eser BE, Barr EW, Frantom PA, Saleh L, Bollinger JM, Krebs C, Fitzpatrick PF (2007) J Am Chem Soc 129:11334–11335
Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L Jr (2003) Science 299:1037–1039
Schroder D, Shaik S, Schwarz H (2000) Acc Chem Res 33:139–145
Shaik S, Chen H, Janardanan D (2011) Nat Chem 3:19–27
McDonald AR, Que L Jr (2013) Coord Chem Rev 257:414–428
Fenton HJH (1894) J Chem Soc 65:899–910
Buda F, Ensing B, Gribnau MCM, Baerends EJ (2003) Chem Eur J 9:3436–3444
Walling C (1998) Acc Chem Res 31:155–157
Pestovsky O, Stoian S, Bominaar EL, Shan X, Münck E, Que L Jr, Bakac A (2005) Angew Chem Int Ed 44:6871–6874
Biswas AN, Puri M, Meier KK, Oloo WN, Rohde GT, Bominaar EL, Munck E, Que L Jr (2015) J Am Chem Soc 137:2428–2431
Bigi JP, Harman WH, Lassalle-Kaiser B, Robles DM, Stich TA, Yano J, Britt RD, Chang CJ (2012) J Am Chem Soc 134:1536–1542
Nam W, Que L Jr (2015) Acc Chem Res 48:2612–2621
Kim S, Cho KB, Lee YM, Chen J, Fukuzumi S, Nam W (2016) J Am Chem Soc 138:10654–10663
Canta M, Rodríguez M, Costas M (2015) Site Sel Catal 372:27–54
Hirao H, Que L Jr, Nam W, Shaik S (2008) Chem Eur J 14:1740–1756
Mandal D, Ramanan R, Usharani D, Janardanan D, Wang BJ, Shaik S (2015) J Am Chem Soc 137:722–733
de Visser SP (2006) J Am Chem Soc 128:15809–15818
de Visser SP (2006) J Am Chem Soc 128:9813–9824
de Visser SP, Nam W (2008) J Phys Chem A 112:12887–12895
Hirao H, Kumar D, Que L Jr, Shaik S (2006) J Am Chem Soc 128:8590–8606
Wang Y, Wang Y, Han K-L (2009) J Biol Inorg Chem 14:533–545
Wang Y, Han K-L (2010) J Biol Inorg Chem 15:351–359
Kumar D, Hirao H, Que L Jr, Shaik S (2005) J Am Chem Soc 127:8026–8027
de Visser SP (2005) J Phys Chem A 109:11050–11057
Shaik S, Kumar D, de Visser SP (2008) J Am Chem Soc 130:10128–10140
Frisch MJ et al (2009) Gaussian 09, revision D.01. Gaussian, Wallingford
Becke AD (1992) J Chem Phys 96:2155–2160
Becke AD (1992) J Chem Phys 97:9173–9177
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hay JP, Wadt WR (1985) J Chem Phys 82:299–310
Friesner RA, Murphy RB, Beachy MD, Ringnalda MN, Pollard WT, Dunietz BD, Cao YX (1999) J Phys Chem A 103:1913–1928
Schaefer A, Horn H, Ahlrichs R (1992) J Chem Phys 97:2571–2577
Schaefer A, Huber C, Ahlrichs R (1994) J Chem Phys 100:5829–5835
Sebastien C, Frederic B, Eric H (2014) J Comp Chem 35:82–93
Roy L (2019) Chem Plus Chem 84:893–906
Manzetti S, Tian L, Behzadi H, Estrafili MD, Thi L (2015) Ha-Linh, Vach H. RSC Advances 5:78192–78208
Hirao H, Kumar D, Thile W, Shaik S (2005) J Am Chem Soc 127:13007–13018
Mukherjee G, Alili A, Barman P, Kumar D, Sastri CV, de Visser SP (2019) Chemistry 25:5086–5098
Amy T, Matthew GQ, Tomasz B, de Visser SP (2018) ACS Catal 8:8685–8698
Mondal B, Roy L, Neese F, Shengfa F (2016) Israel J Chem 56:768–772
Saouma CT, Mayer JM (2014) Chem Sci 5:21–31
Kumar D, Latifi R, Kumar S, Rybak-Akimova EV, Sainna MA, de Visser SP (2013) Inorg Chem 52:7968–7979
Kumar D, Karamzadeh B, Sastry GN, de Visser SP (2010) J Am Chem Soc 132:7659–7667
Bae SH, Seo MS, Lee Y-M, Cho K-B, Kim W-S, Nam W (2016) Angew Chem Int Ed 55:8027–8031
Klein JE, Dereli B, Que L Jr, Cramer CJ (2016) Chem Commun 52:10509–10512
Lefebvre C, Rubez G, Khartabil H, Boisson JC, Contreras-Garcia J, Henon E (2017) Phys Chem Chem Phys 19:17928–17936
Tian L, Feiwu C (2012) J Comput Chem 33:580–592
Acknowledgements
This work was supported by Natural Science Foundation of Liaoning Province (Grant 20180550765), the Open Project of SKLMRD (Open Project of State Key Laboratory of Molecular Reaction Dynamics), and National Natural Science Foundation of China (Grant 31771914). The results of quantum chemical calculations described in this paper were obtained on the homemade Linux cluster of group 1101, Dalian Institute of Chemical Physics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Wang, Y., Li, W. et al. The oxidation of cyclo-olefin by the S = 2 ground-state complex [FeIV(O)(TQA)(NCMe)]2+. J Biol Inorg Chem 25, 371–382 (2020). https://doi.org/10.1007/s00775-020-01768-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01768-1