Abstract
Aims
Many soil scientists think that soil phosphate exists as discrete compounds of iron, aluminium and calcium and, accordingly, use chemical fractionation schemes to identify these compounds.
Methods
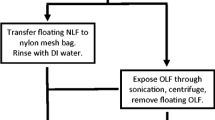
We reacted a sample of goethite and a sample of aluminium oxide with a phosphate solution under conditions chosen to facilitate penetration of phosphate. Thus the sample of goethite had neither calcium nor aluminium present and similarly the sample of aluminium oxide had neither iron nor calcium. We included a sample of hydroxyapatite which had neither iron nor aluminium present. We subjected the samples to two fractionation procedures; the original Chang and Jackson (1957) method and a variant of it.
Results
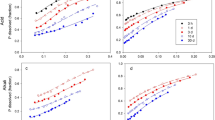
For the phosphated goethite and aluminium oxide, energy dispersive X-ray spectra did not detect any discrete aluminium or iron phosphates; dissolution studies were consistent with penetration of phosphate. Both fractionation procedures detected discrete compounds even though none were present. They also detected iron, aluminium and calcium phosphates for samples for which they were not present. We also critically discuss other evidence for the existence of discrete iron, aluminium and calcium phosphates in soils.
Conclusions
Fractionation procedures designed to measure chemically specified phosphate fractions in soil are fallacious and should be abandoned.






Similar content being viewed by others
References
Alobeedallah H, Ellis JL, Rohanizadeh R, Coster H, Dehghani F (2011) Preparation of nanostructured hydroxyapatite in organic solvents for clinical applications. Trends Biomater Artif Organs 25:12–19
Barrow NJ (1999) The four laws of soil chemistry: the Leeper lecture 1998. Aust J Soil Res 37:787–829
Barrow NJ (1972) Influence of solution concentration of calcium on the adsorption of phosphate, sulfate and molybdate by soils. Soil Sci 113:175–180
Barrow NJ (1983) A mechanistic model for describing the sorption and desorption of phosphate by soil. J Soil Sci 34:733─750
Barrow NJ, Barman P, Debnath A (2018) Three residual benefits of applying phosphate fertilizer. Soil Sci Soc Am J 82:1168–1176
Barrow NJ, Bowden JW (1987) A comparison of models for describing the adsorption of anions on a variable charge mineral surface. J Colloid Interface Sci 119:236–250
Barrow NJ, Debnath A (2014) Effect of phosphate status on the sorption and desorption properties of some soils of northern India. Plant Soil 378:383–395
Barrow NJ, Debnath A (2015) Effect of phosphate status and pH on sulphate sorption and desorption. Europ J Soil Sci 66:286–297
Barrow NJ, Debnath A, Chattergee S (2016) Effect of pH and prior phosphate application on the reaction of fluoride with soils from northern India. Europ J Soil Sci 67:294–230
Barrow NJ, Ellis AS (1986) Testing a mechanistic model 3. The effects of pH on fluoride retention by a soil. J Soil Sci 37:287–293
Barrow NJ, Shaw TC (1979) Effect of ionic strength and nature of the cation on desorption of phosphate from soil. J Soil Sci 30:53–65
Beauchemin S, Hesterberg D, Chou J, Beauchemin M, Simard RR, Sayers DE (2003) Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray absorption near-edge structure spectroscopy and chemical fractionation. J Environ Qual 32:1809–1819
Bowden JW, Posner AM, Quirk JP (1977) Ionic adsorption on variable charge mineral surfaces. Theoretical charge development and titration curves. Aust J Soil Res 15:121–136
Chang SC, Jackson ML (1957) Fractionation of soil phosphorus. Soil Sci 84:133–144
Eriksson AK, Gustafsson JP, Hesterberg D (2015) Phosphorus speciation of clay fractions from long-term fertility experiments in Sweden. Geoderma 241:68–74
Gu C, Dam T, Hart S, Turner B. L, Chadwick O, Berhe A. A, Hu Y, Zhu M (2020) Quantifying uncertainties in sequential chemical extraction of soil phosphorus using XANES spectroscopy. Env Sc Just accepted
Hall AD, Amos A (1906) XXVII.—the determination of available plant food in soils by the use of weak acid solvents. Part II. J Chem Soc Trans 89:205–222
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation soil practices and by laboratory incubations. Sci Soc Am J 46:970–976
Lindsay WL (1979) Chemical equilibria in soils. Wiley
Marcus Y (1988) Ionic radii in aqueous solutions. Chem Rev 88:1475–1498
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Penn CJ, Camberto JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120–138
Petrícek V, Dušek M, Palatinus L (2014) Crystallographic computing system JANA2006: general features. Zeitschrift für Kristall - Crystalline Materials 229:345–352. https://doi.org/10.1515/zkri-2014-1737
Posner AM, Barrow NJ (1982) Simplification of a model for ion adsorption on oxide surfaces. J Soil Sci 33:211–231
Price G (ed) (2006) Australian soil fertility manual, 3rd edition, fertilizer industry Federation of Australia and CSIRO, p 45
Prietzel J, Dümig A, Wu Y, Zhou J, Klysubun W (2013) Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim Cosmochim Acta 108:154–171
Russell EJ, Prescott JA (1916) The reaction between dilute acids and the phosphorus compounds of the soil. J Agric Sci 8:65–110
Shirai T, Watanabe H, Fuji M, Takahasi M (2009) Structural properties and surface characteristics on aluminium oxide powders. Ann Rep Ceram Res 9:23–31
Shainberg I, Kemper WD (1966) Hydration status of adsorbed cations. Proc Soil Sci Soc Amer 30:707–713
Shainberg I, Kemper WD (1967) Ion exchange equilibrium on montmorillonite. Soil Sc 103:4–9
Strauss R, Brümmer GW, Barrow NJ (1997) Effects of crystallinity of goethite II rates of sorption and desorption of phosphate. Europ J Soil Sci 48:101–114
Yoldas BE (1973) Hydrolysis of aluminium alkoxides and bayerite conversion. J Appl Chem Biotechnol 23:803–809
Zhang H, Kovar JL (2009) Fractionation of soil phosphorus. In: Kovar JL, Pierzyhski GM (eds) Methods of phosphorus analysis in soils, 2nd edn. North Carolina State University, Raleigh, pp 50–60
Acknowledgements
The authors acknowledge Dr. Partha Pratim Jana for his help and support. Nilanjan Roy acknowledges CRF, IIT Kharagpur for instrumental facility and CSIR for a junior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 515 kb)
Rights and permissions
About this article
Cite this article
Barrow, N.J., Sen, A., Roy, N. et al. The soil phosphate fractionation fallacy. Plant Soil 459, 1–11 (2021). https://doi.org/10.1007/s11104-020-04476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04476-6